This document discusses several key concepts in thermochemistry and thermodynamics:
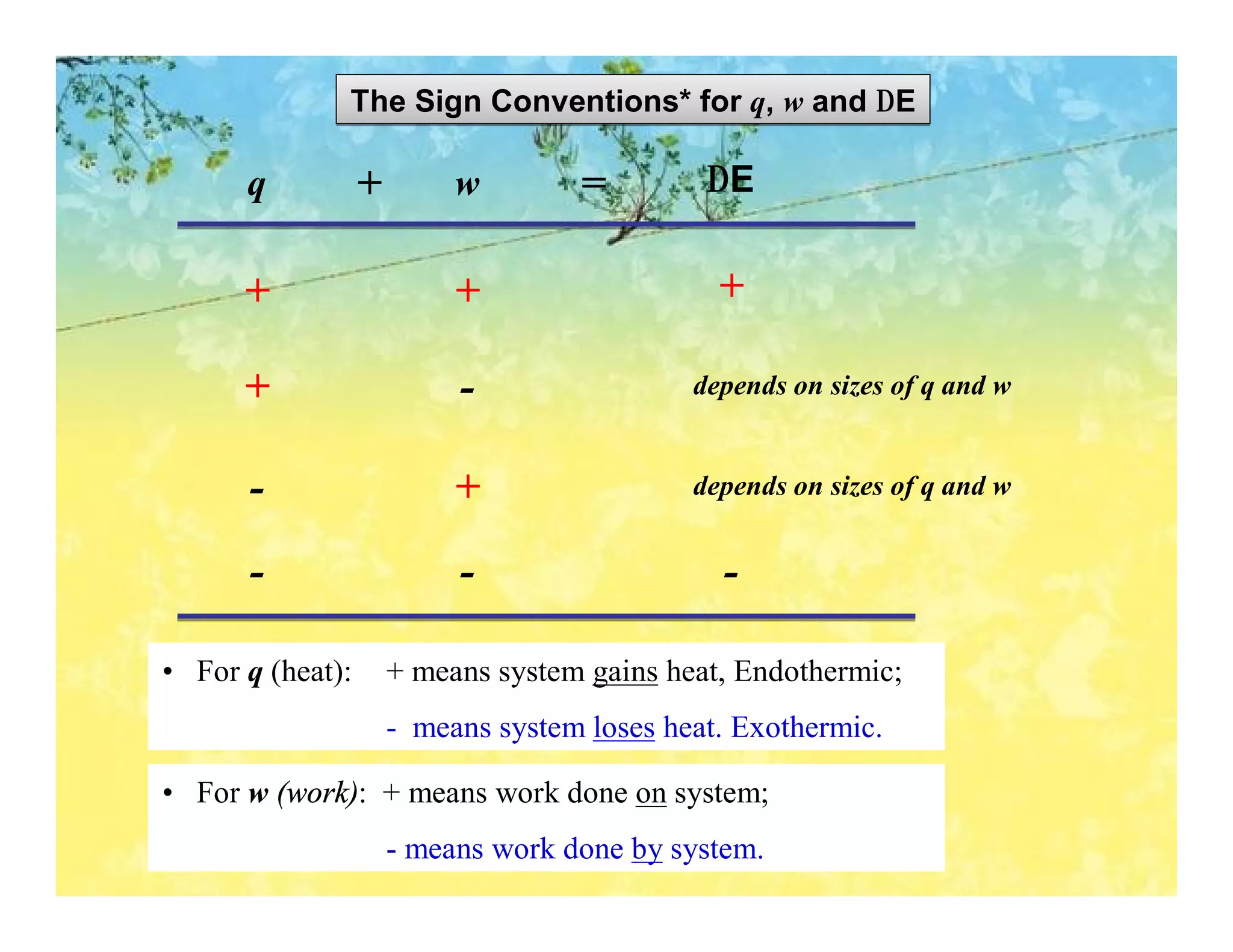
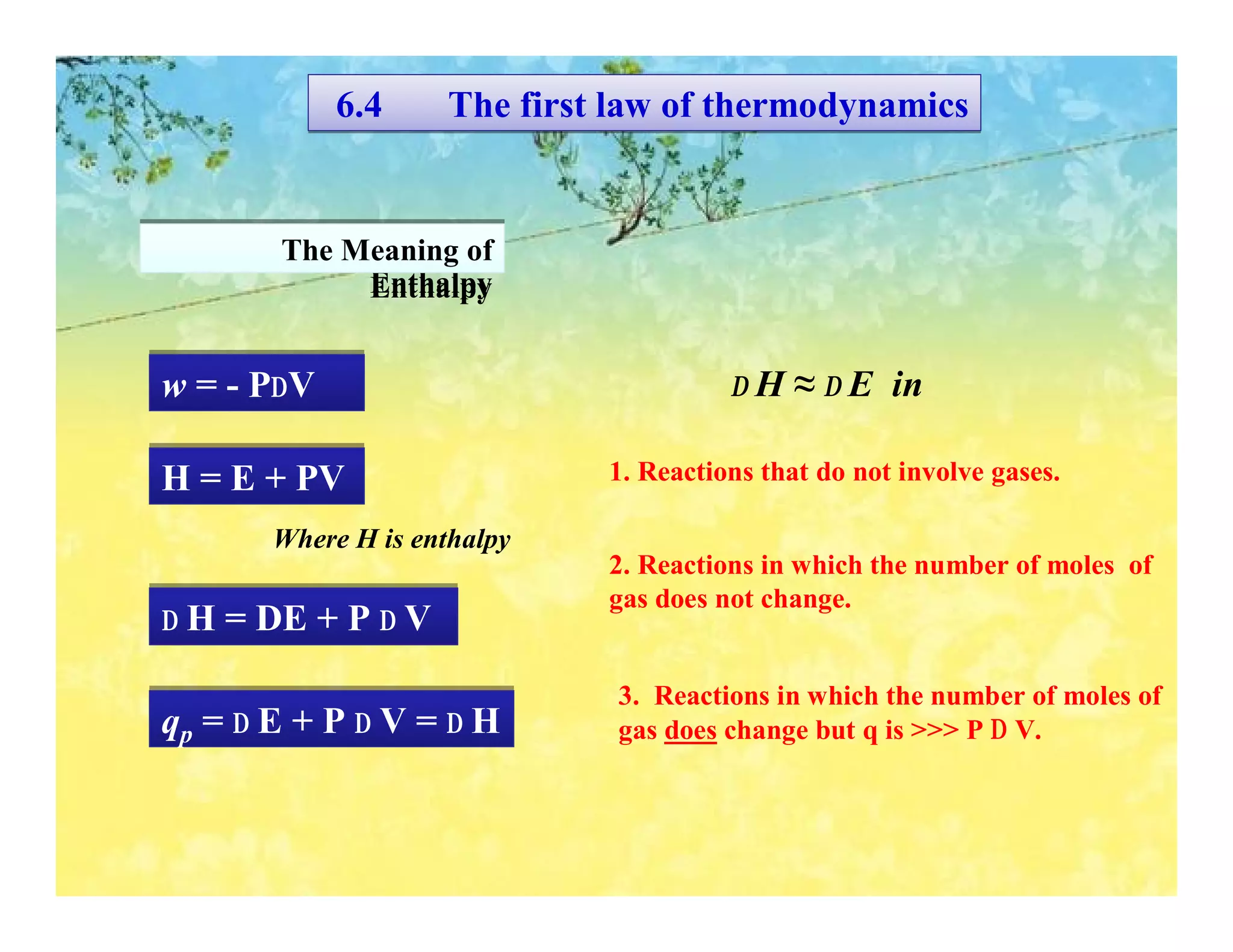
- The first law of thermodynamics relates heat, work, and changes in internal energy. Enthalpy is a useful state function for chemical reactions.
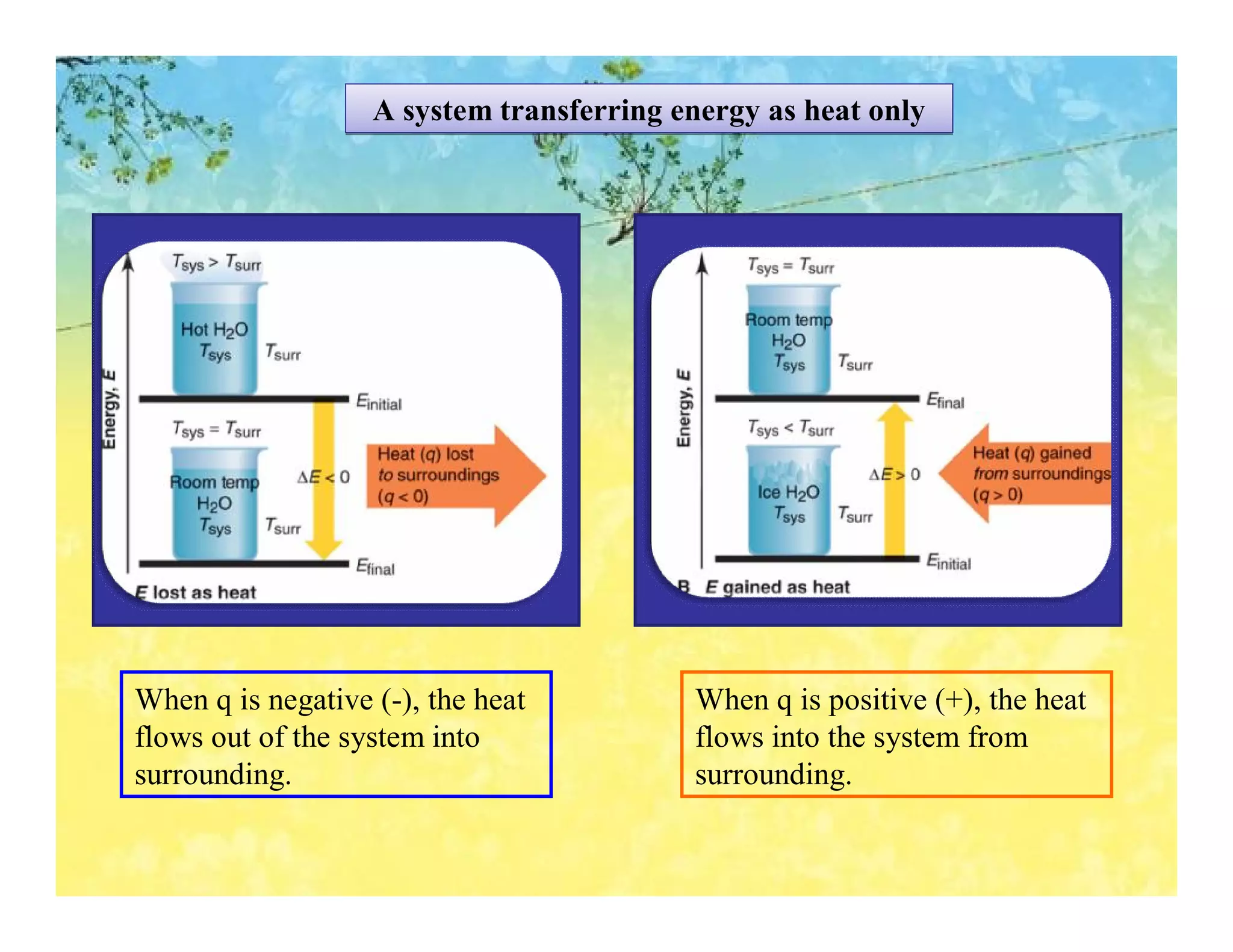
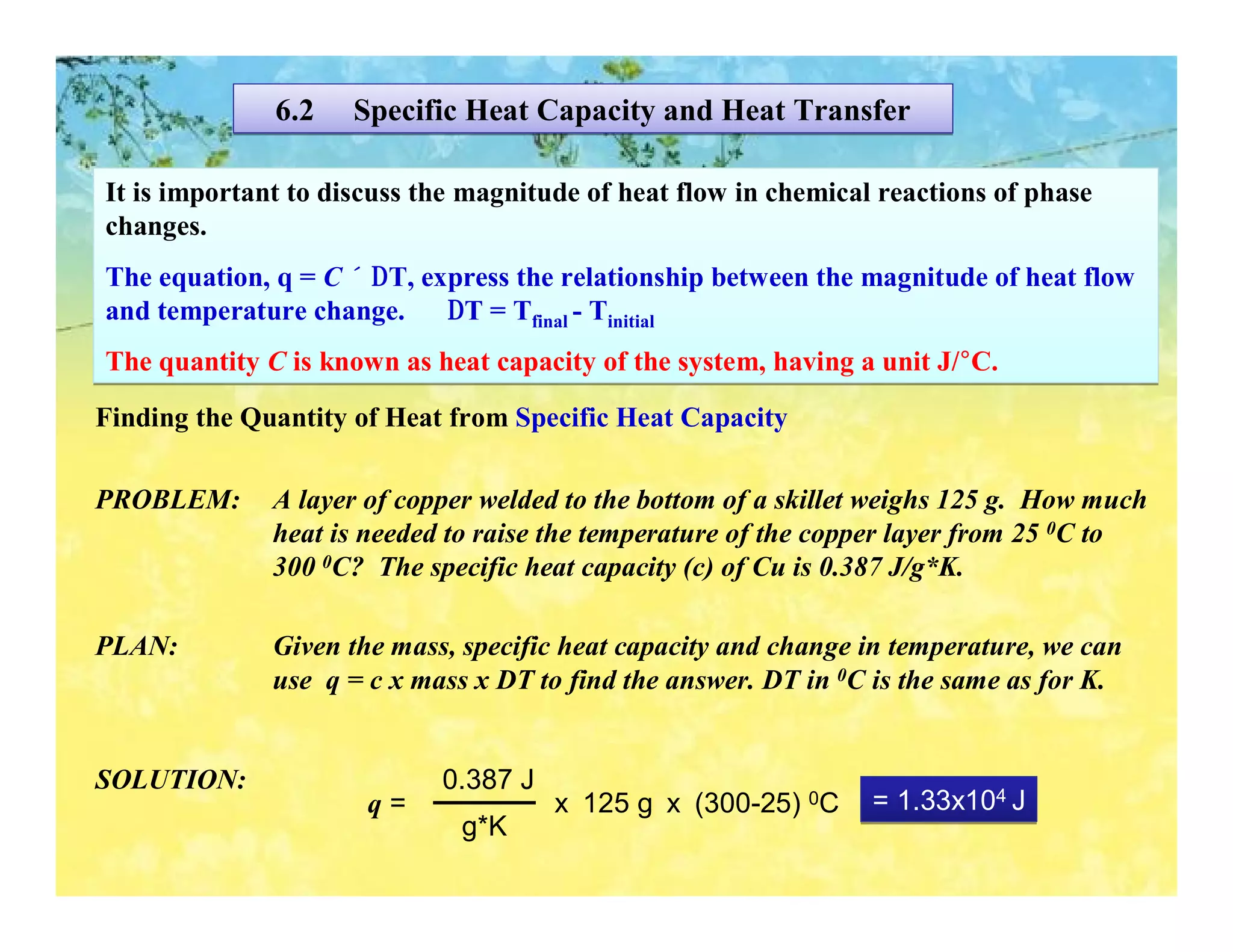
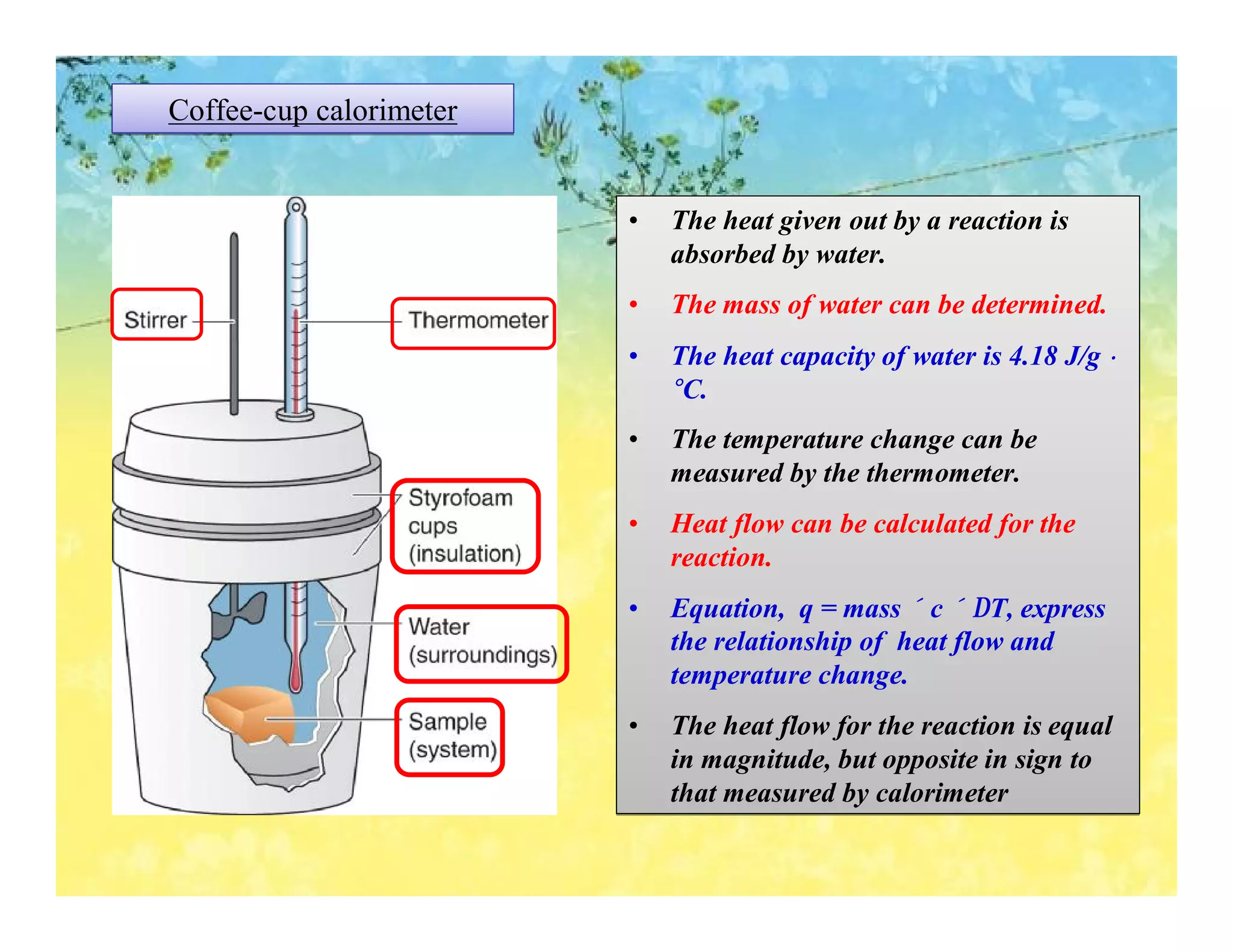
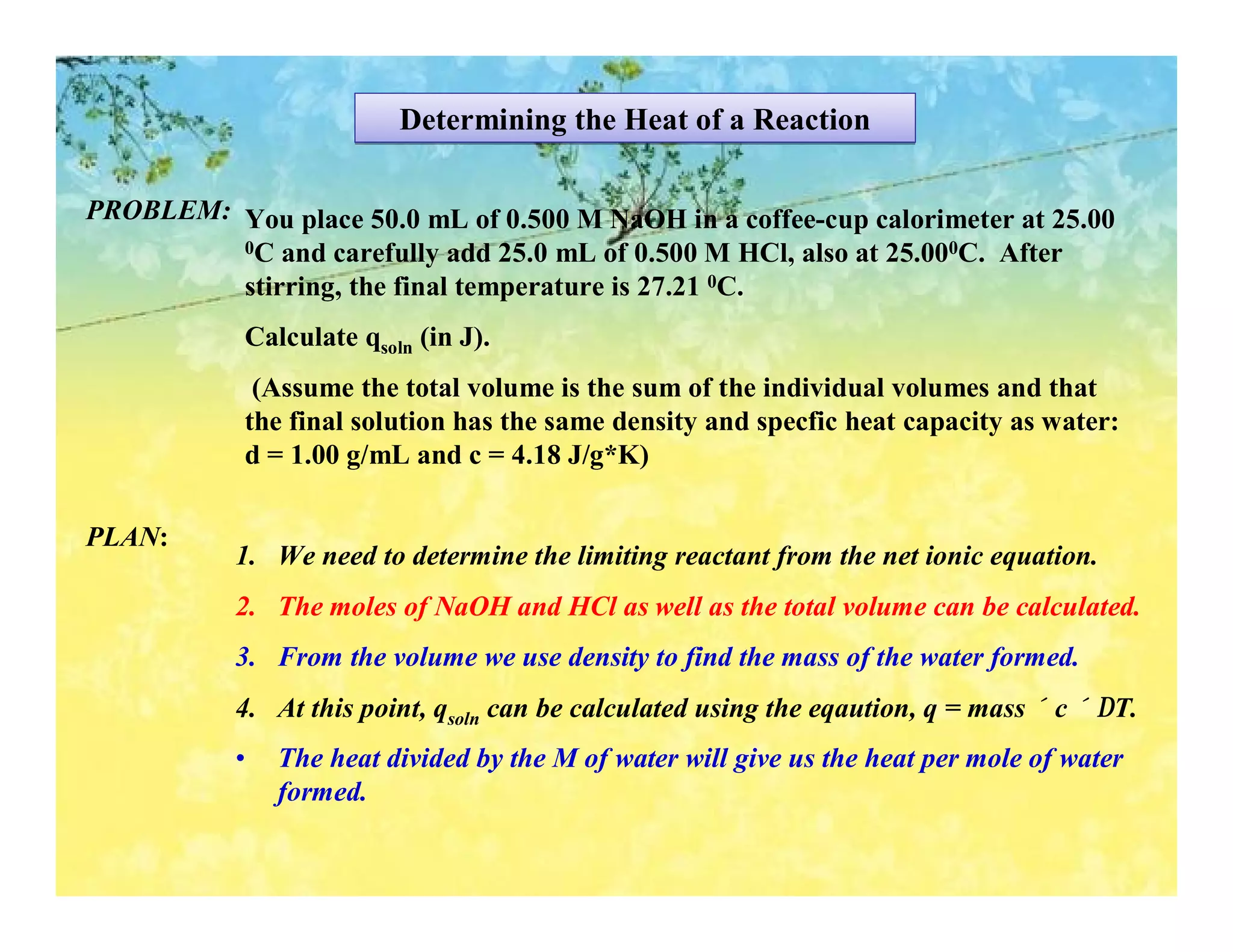
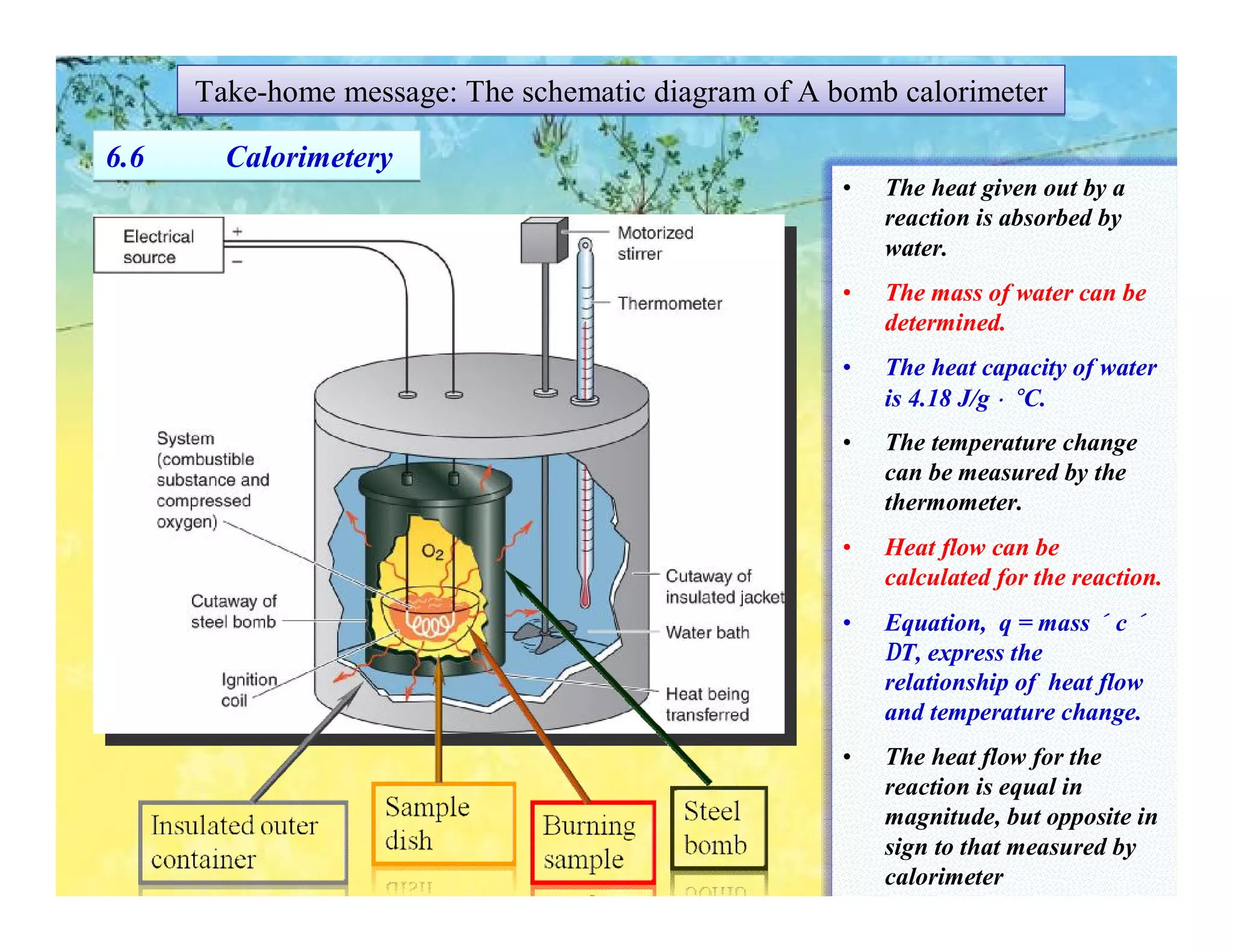
- Calorimetry can be used to measure heat flows and determine enthalpy changes during chemical and physical processes. Bomb calorimetry and coffee cup calorimetry are described.
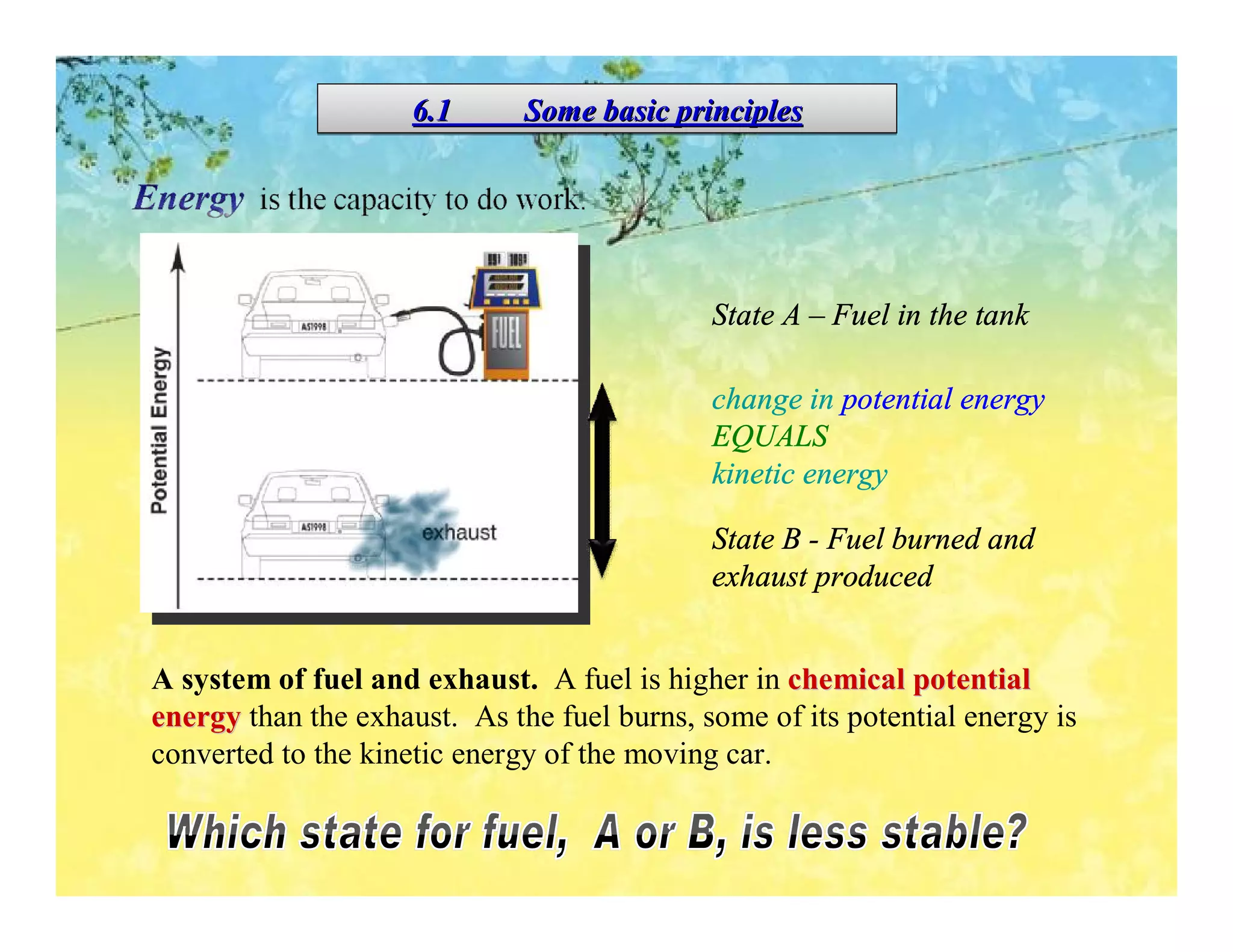
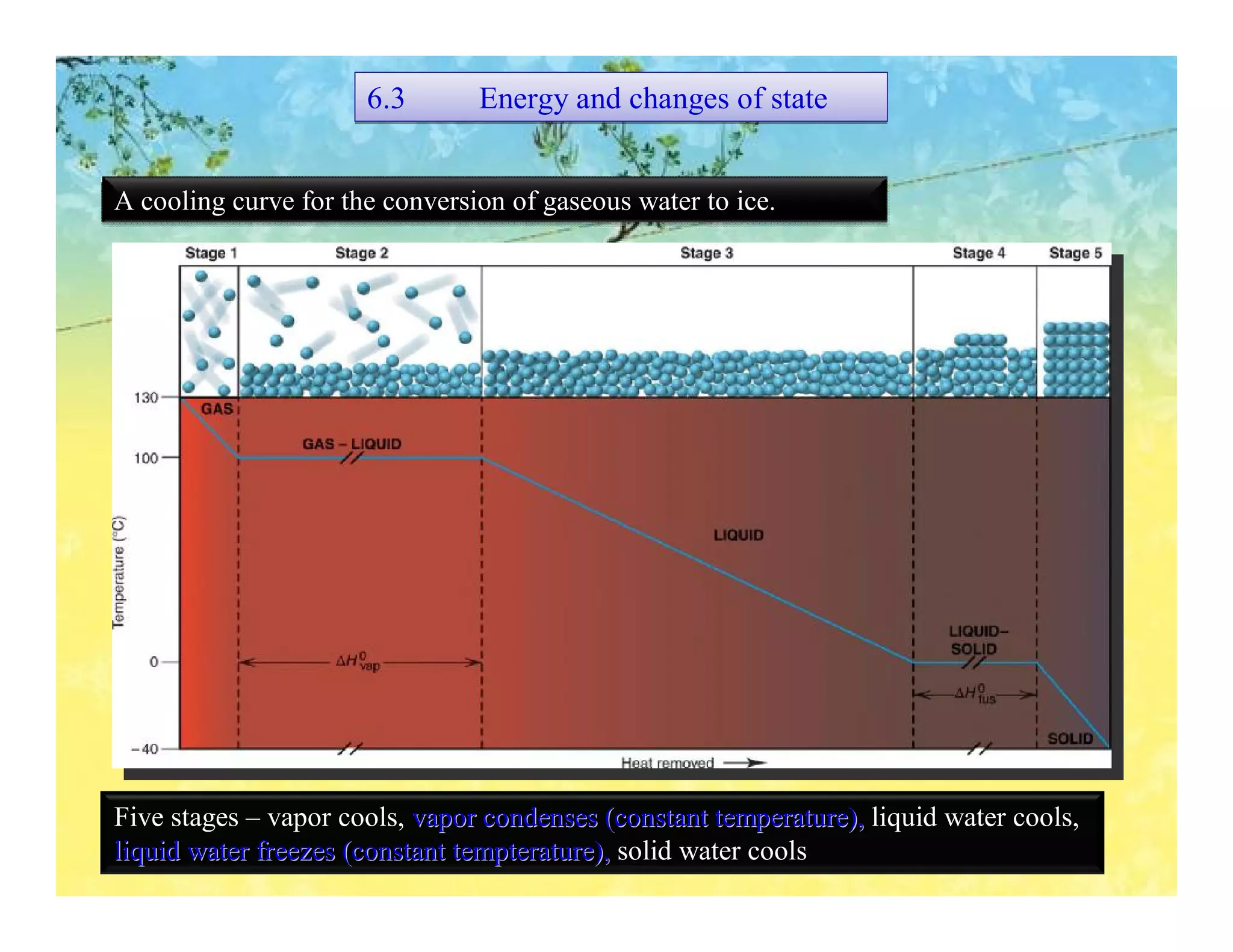
- Enthalpy changes accompany chemical reactions and phase changes. The sign and magnitude of enthalpy changes can indicate whether a process is exothermic or endothermic.
- Hess's law allows calculation of enthalpy changes from thermochemical data using stepwise processes













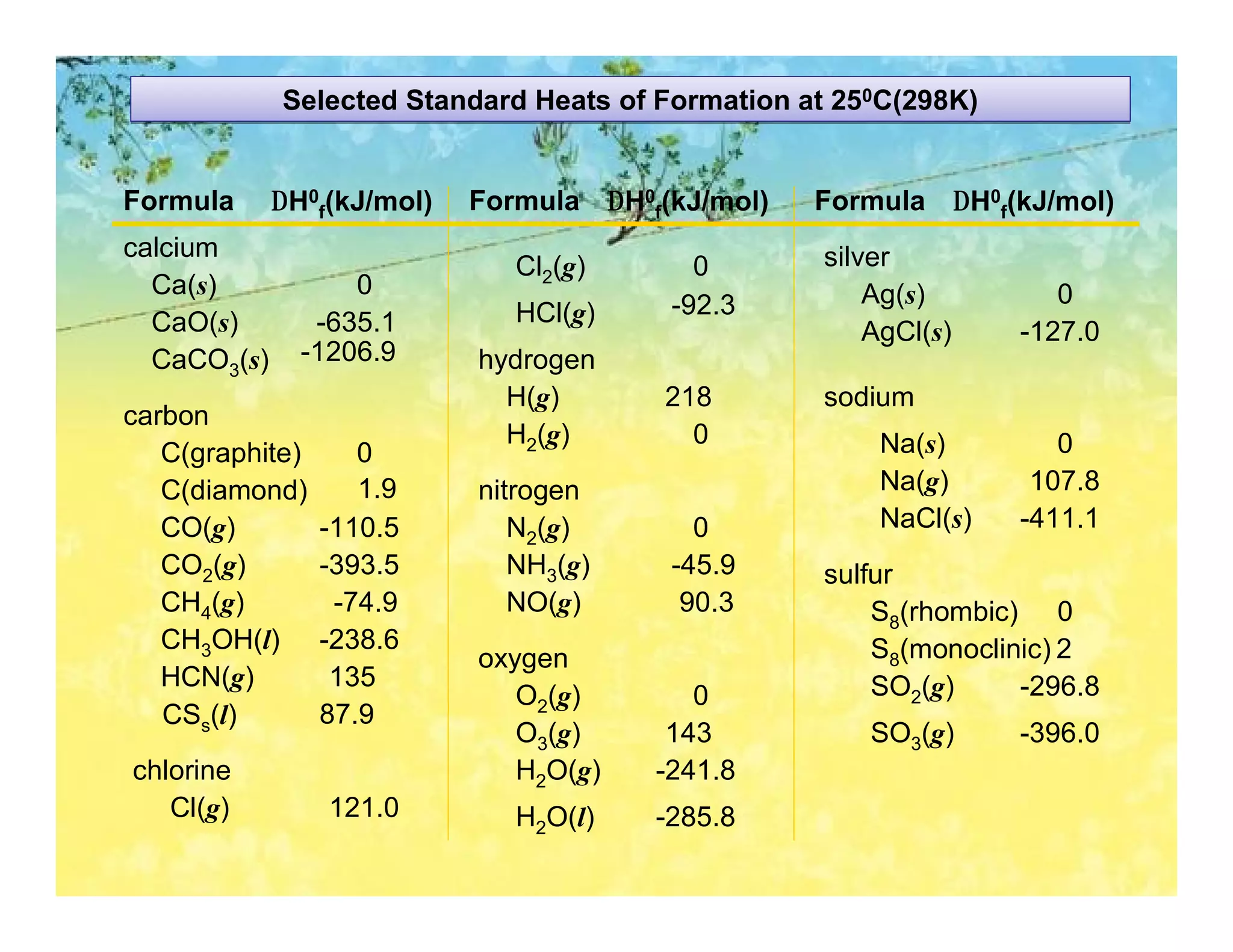
![Calculating the Heat of Reaction from Heats of Formation
PROBLEM: Nitric acid, whose worldwide annual production is about 8 billion
kilograms, is used to make many products, including fertilizer, dyes, and
explosives. The first step in the industrial production process is the
oxidation of ammonia:
4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
Calculate DH0rxn from DH 0f values.
PLAN: Look up the DH0f values and use Hess’s Law to find DHrxn.
SOLUTION:
DHrxn = S mDH0f (products) - S nDH0f (reactants)
DHrxn = [4(DH0f NO(g) + 6(DH0f H2O(g)] - [4(DH0f NH3(g) + 5(DH0f O2(g)]
= (4 mol)(90.3 kJ/mol) + (6 mol)(-241.8 kJ/mol) -
[(4 mol)(-45.9 kJ/mol) + (5 mol)(0 kJ/mol)]
∆Hrxn = -906 kJ
Lectrue 3](https://image.slidesharecdn.com/ch6-thermo-091013121140-phpapp02/75/Thermochemistry-25-2048.jpg)