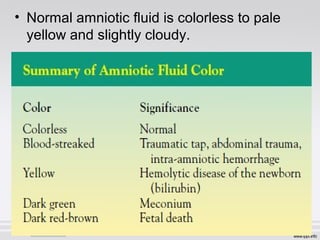
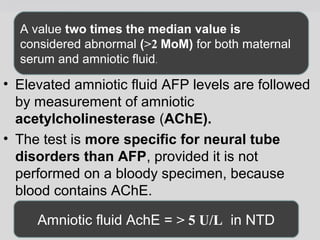
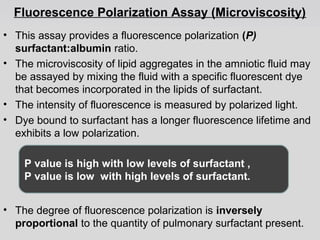
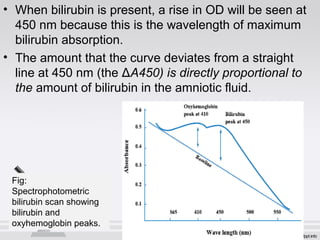
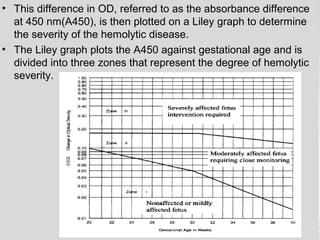
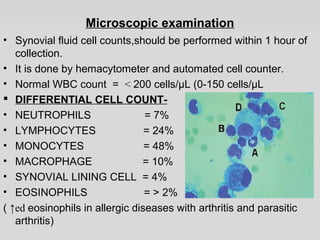
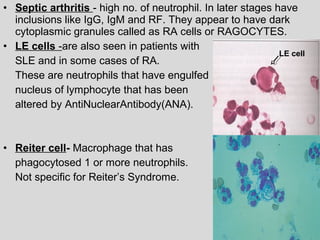
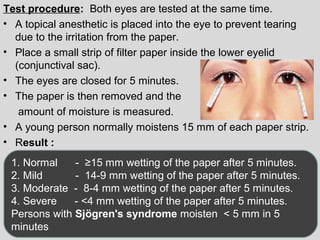
The document provides an extensive overview of body fluid analysis, particularly focusing on amniotic fluid, semen, and other bodily fluids. It discusses collection procedures, testing methodologies, and the clinical significance of different fluid analyses for diagnoses such as genetic disorders, fetal lung maturity, and male infertility. Key concepts include the roles and characteristics of various fluids, physiological impacts of imbalances, and laboratory procedures for testing.