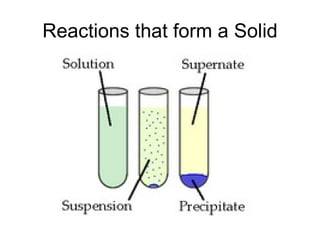
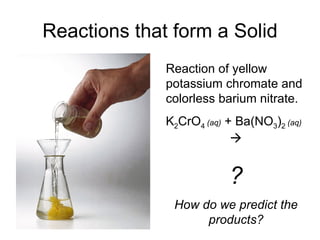
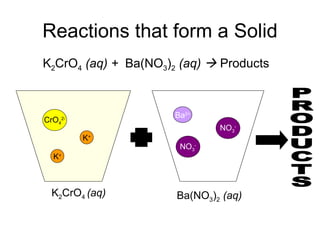
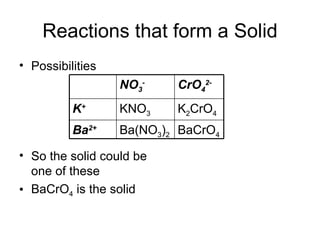
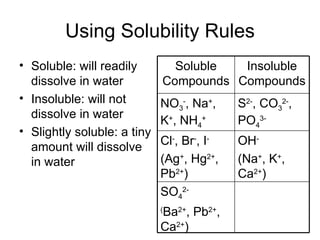
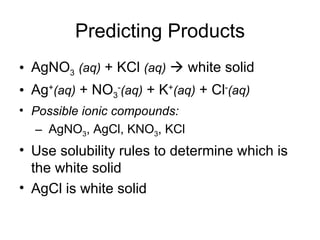
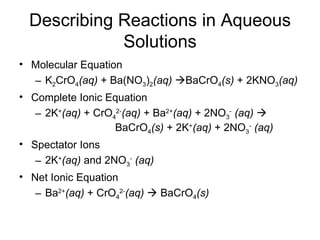
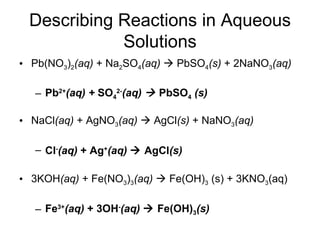
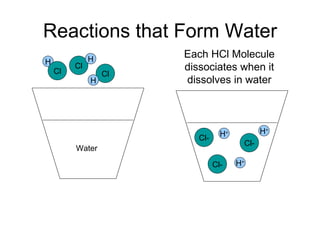
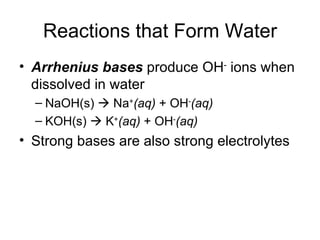
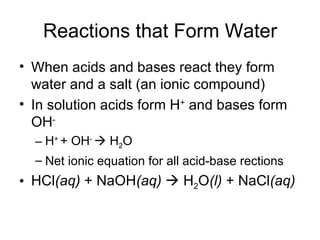
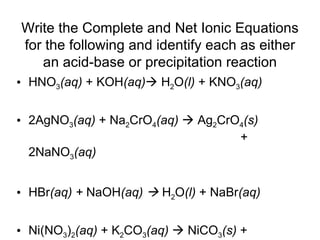
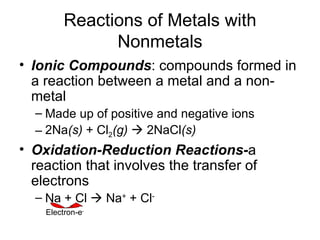
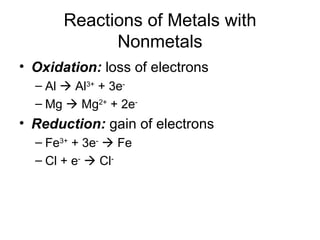
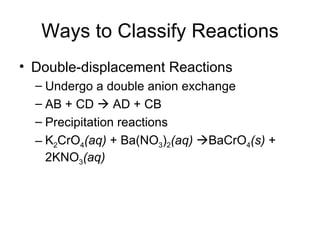
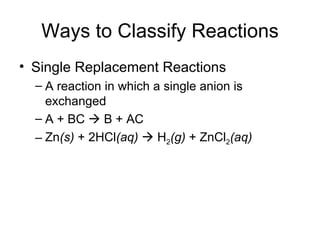
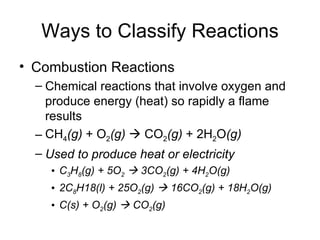
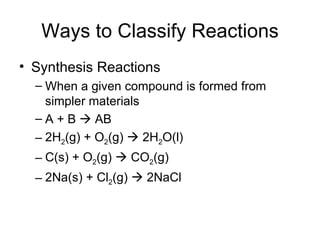
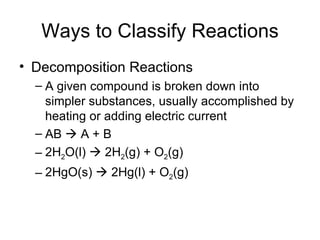
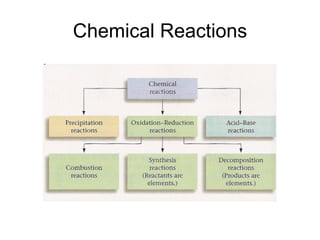
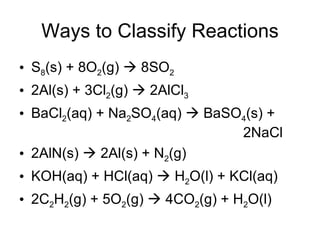
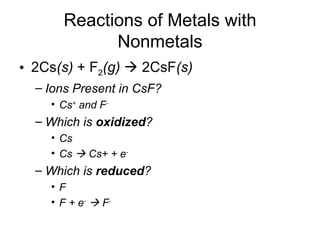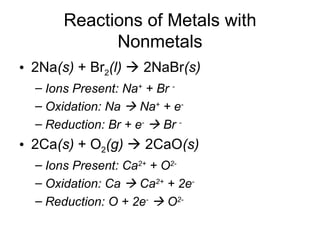This chapter discusses different types of chemical reactions in aqueous solutions. It introduces driving forces that cause reactions, such as formation of a solid, water, or gas. It explains how to predict products using solubility rules and oxidation-reduction reactions when metals react with nonmetals. Reactions are classified into double displacement, acid-base, single replacement, combustion, synthesis, or decomposition reactions based on their driving forces.