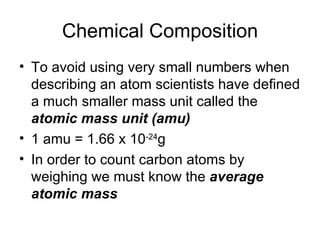
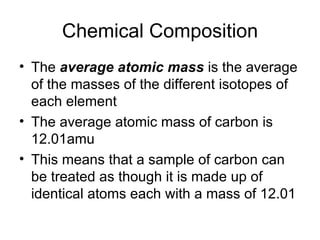
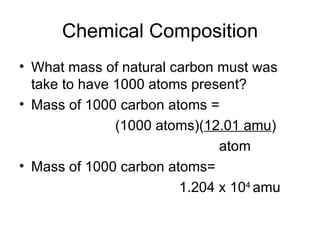
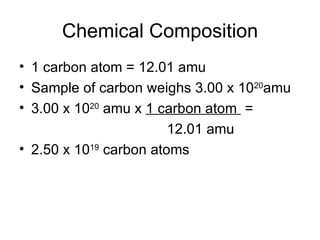
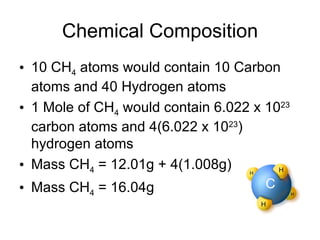
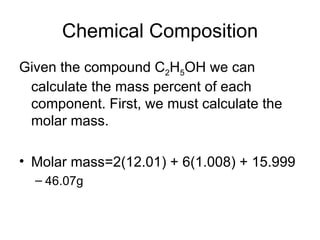
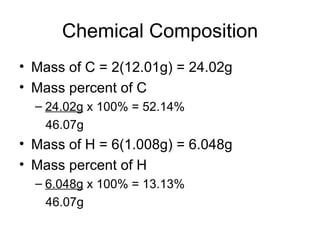
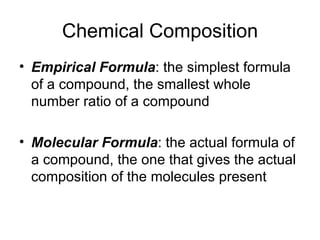
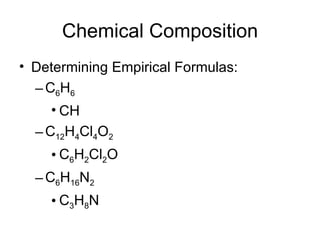
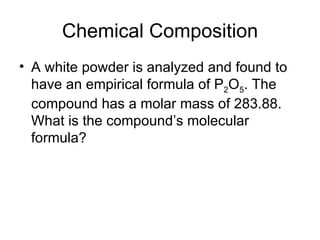
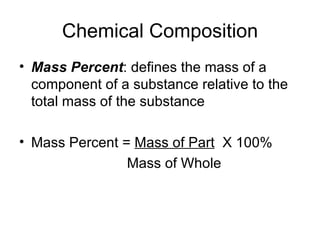The document discusses several chemistry concepts including:
- Calculating average mass and ratios using sample masses of jelly beans and mints.
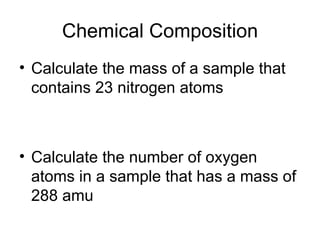
- Using atomic mass units (amu) to count atoms by weighing samples and determining the number of atoms in samples of various elements.
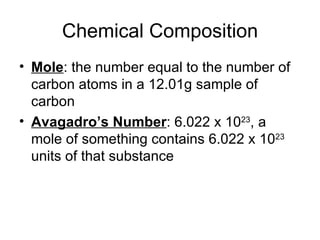
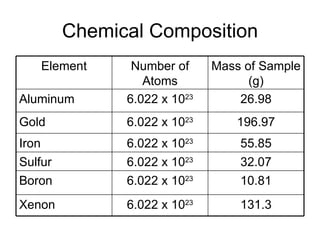
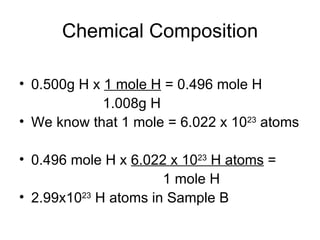
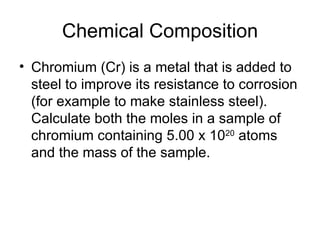
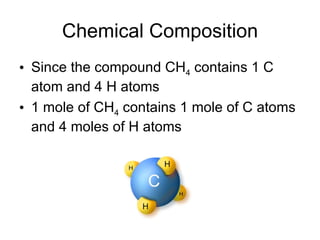
- Defining the mole as 6.022x10^23 units of a substance, and using moles to calculate the number of atoms in samples.
- Calculating molar mass and using molar mass to determine the mass of samples containing a given number of moles.