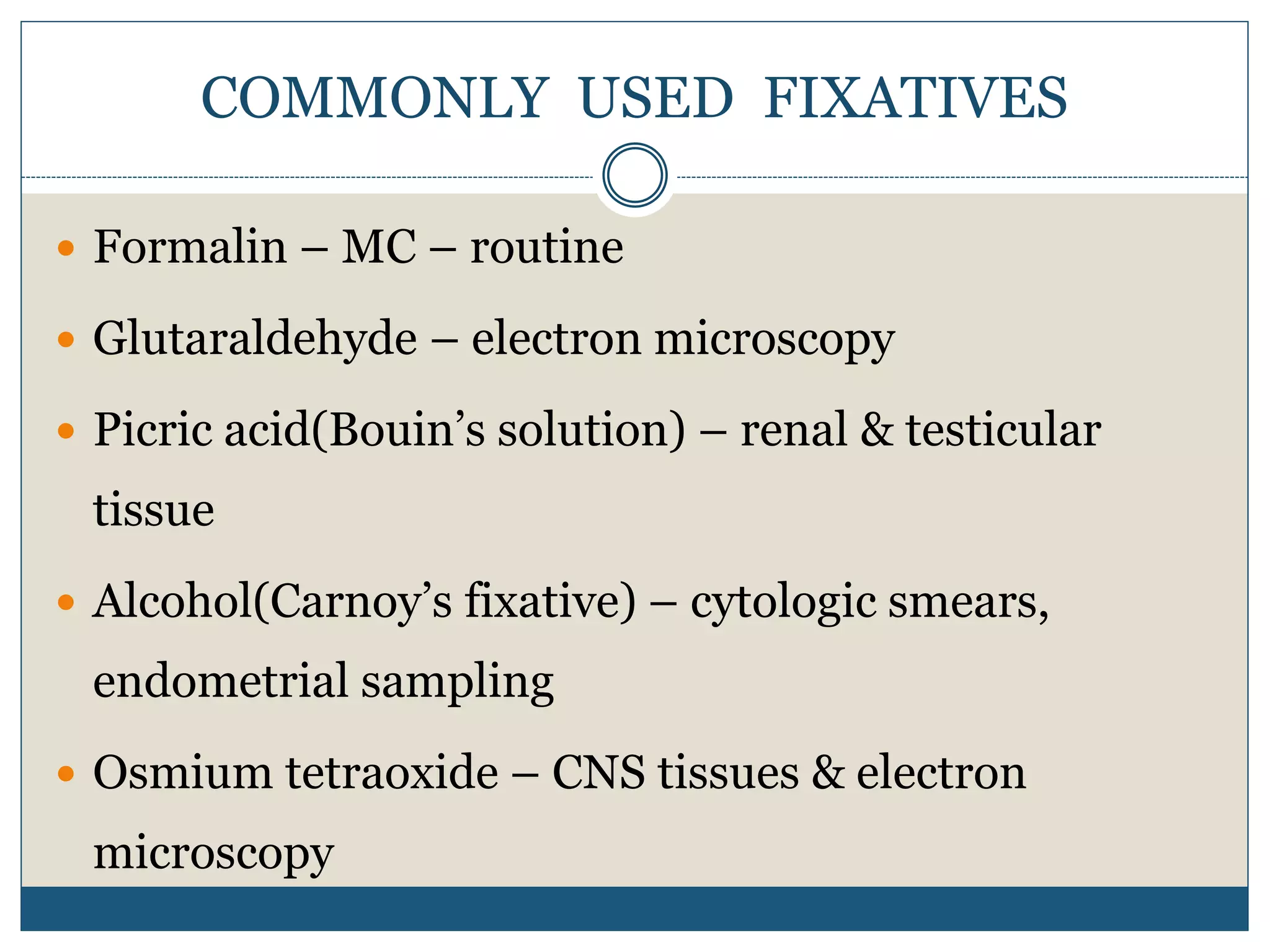
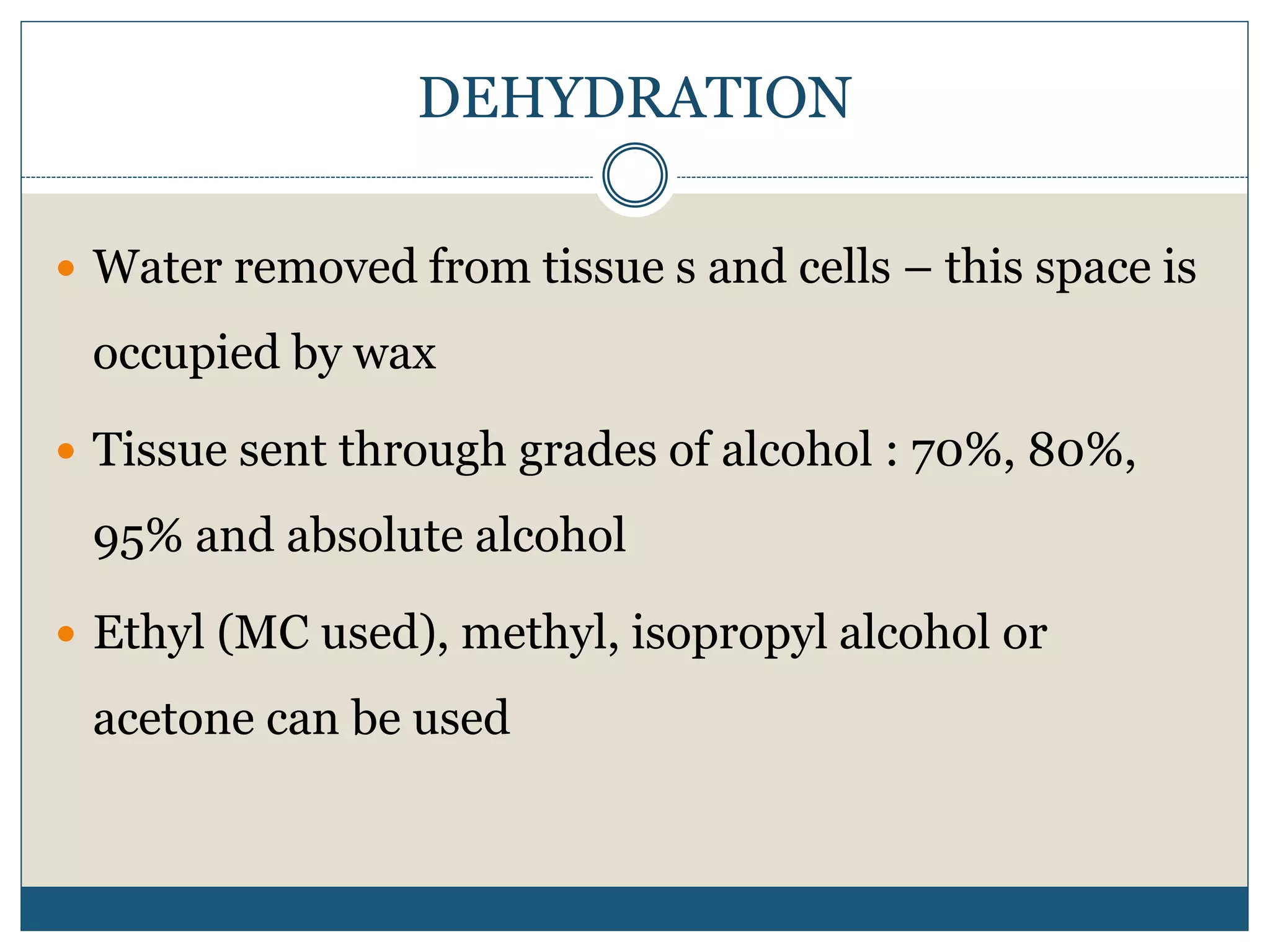
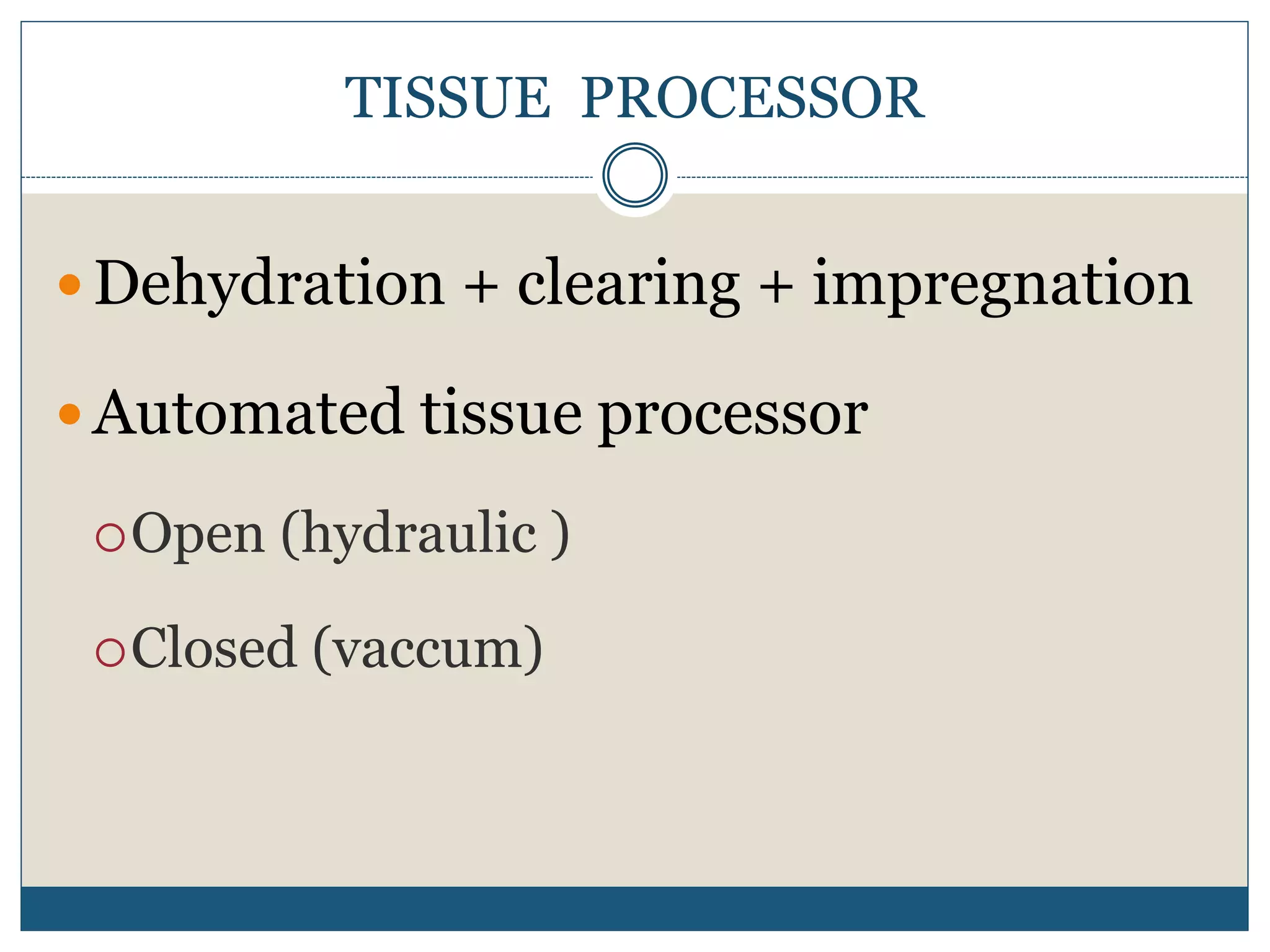
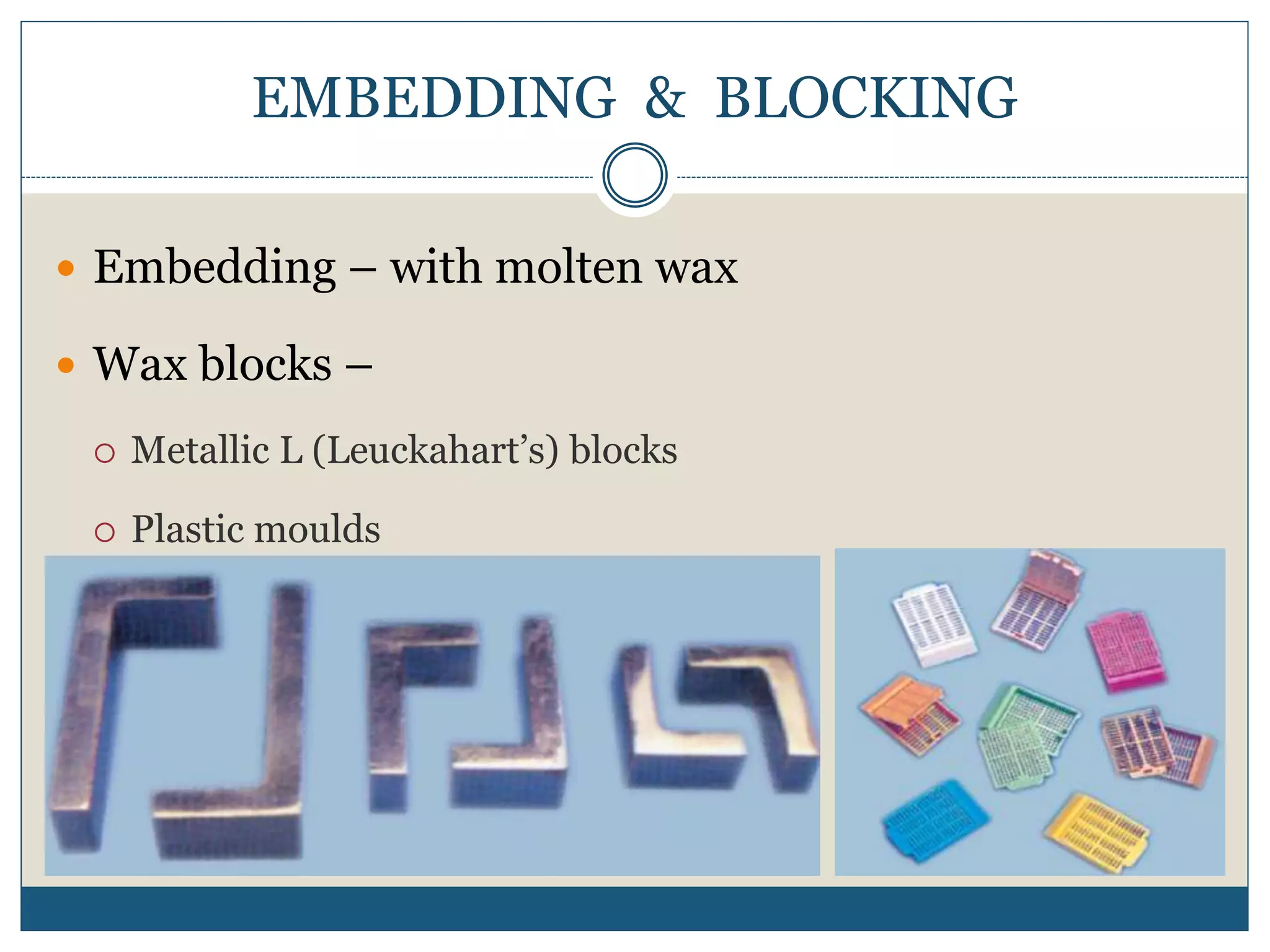
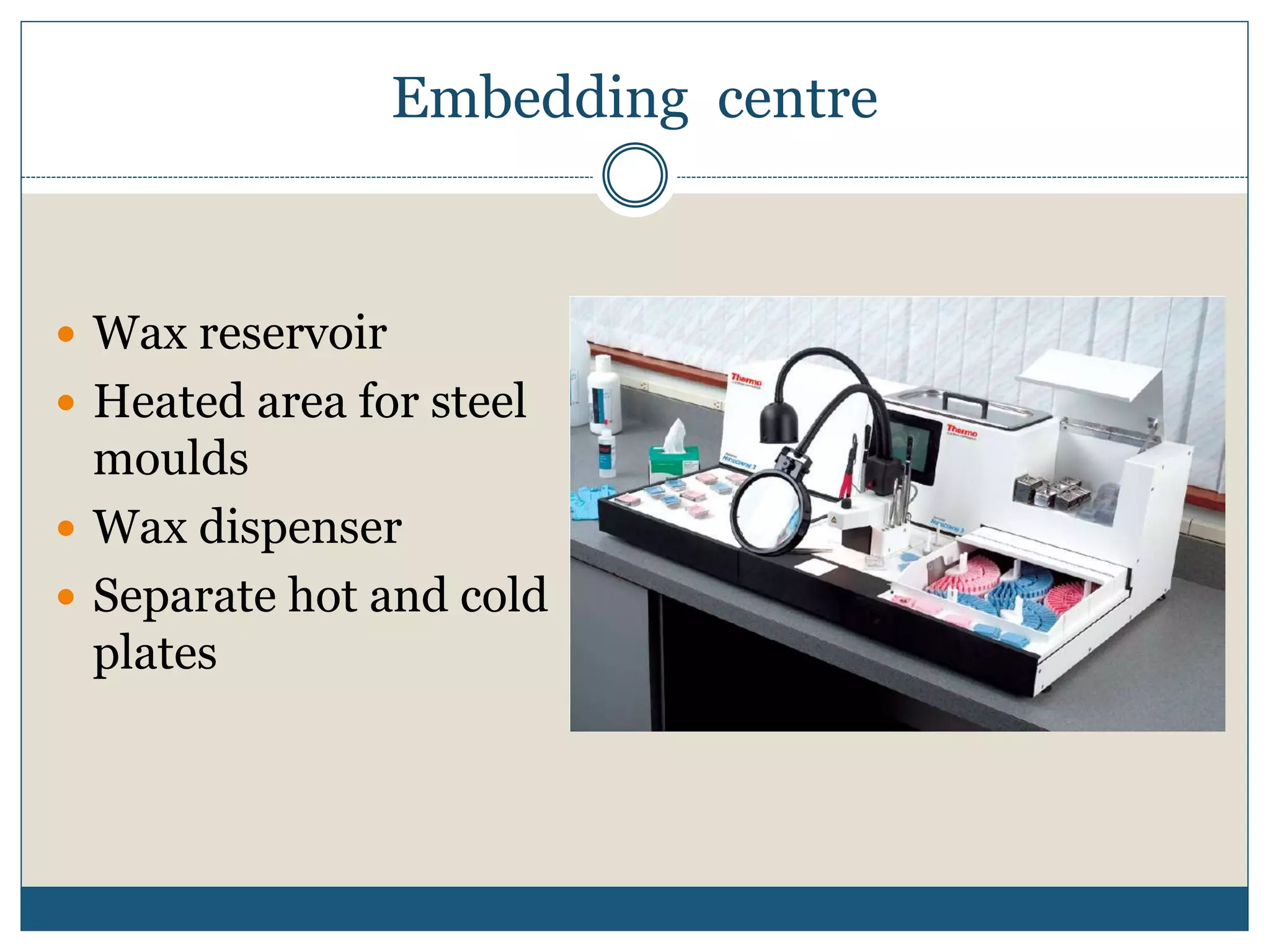
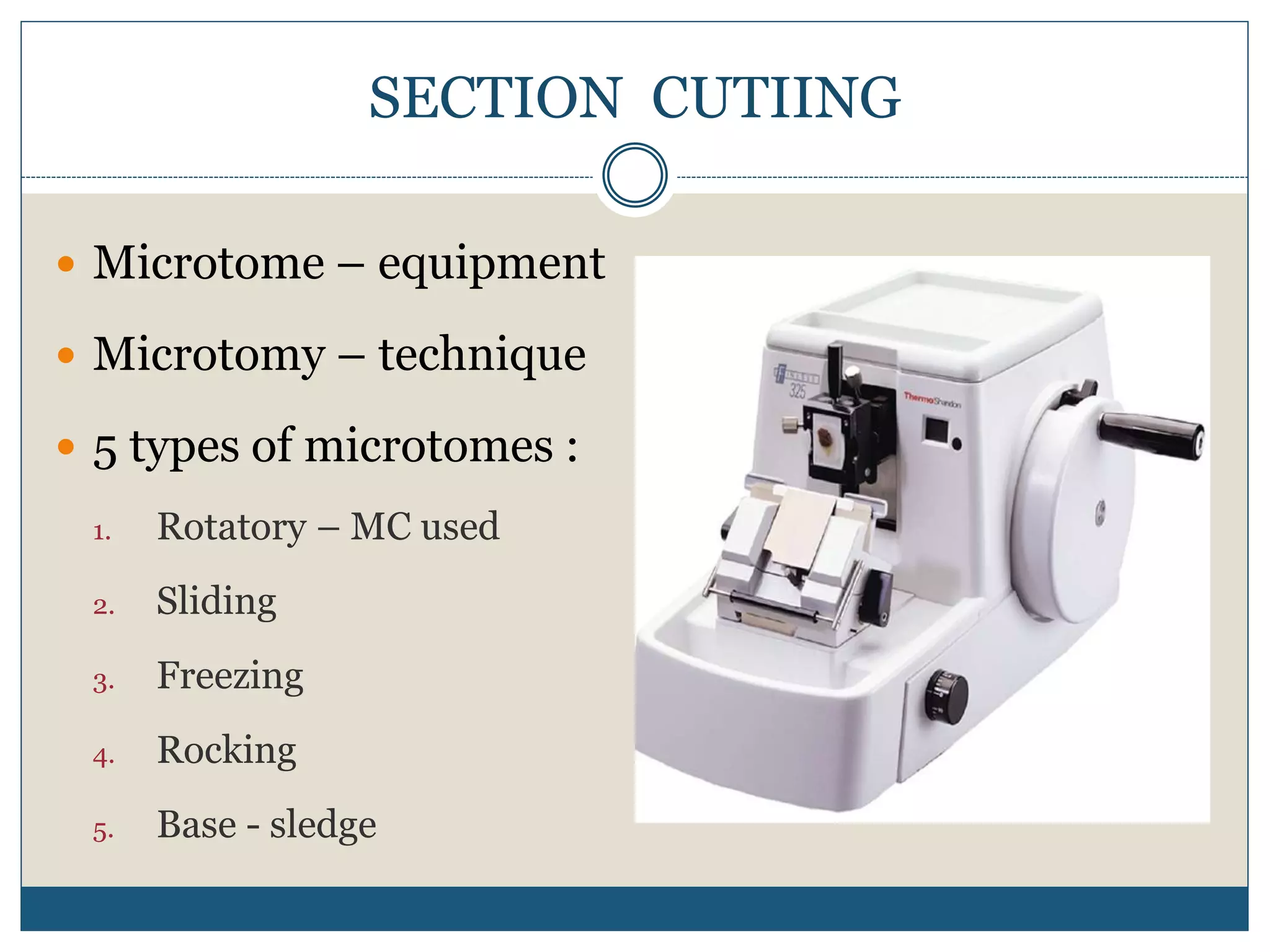
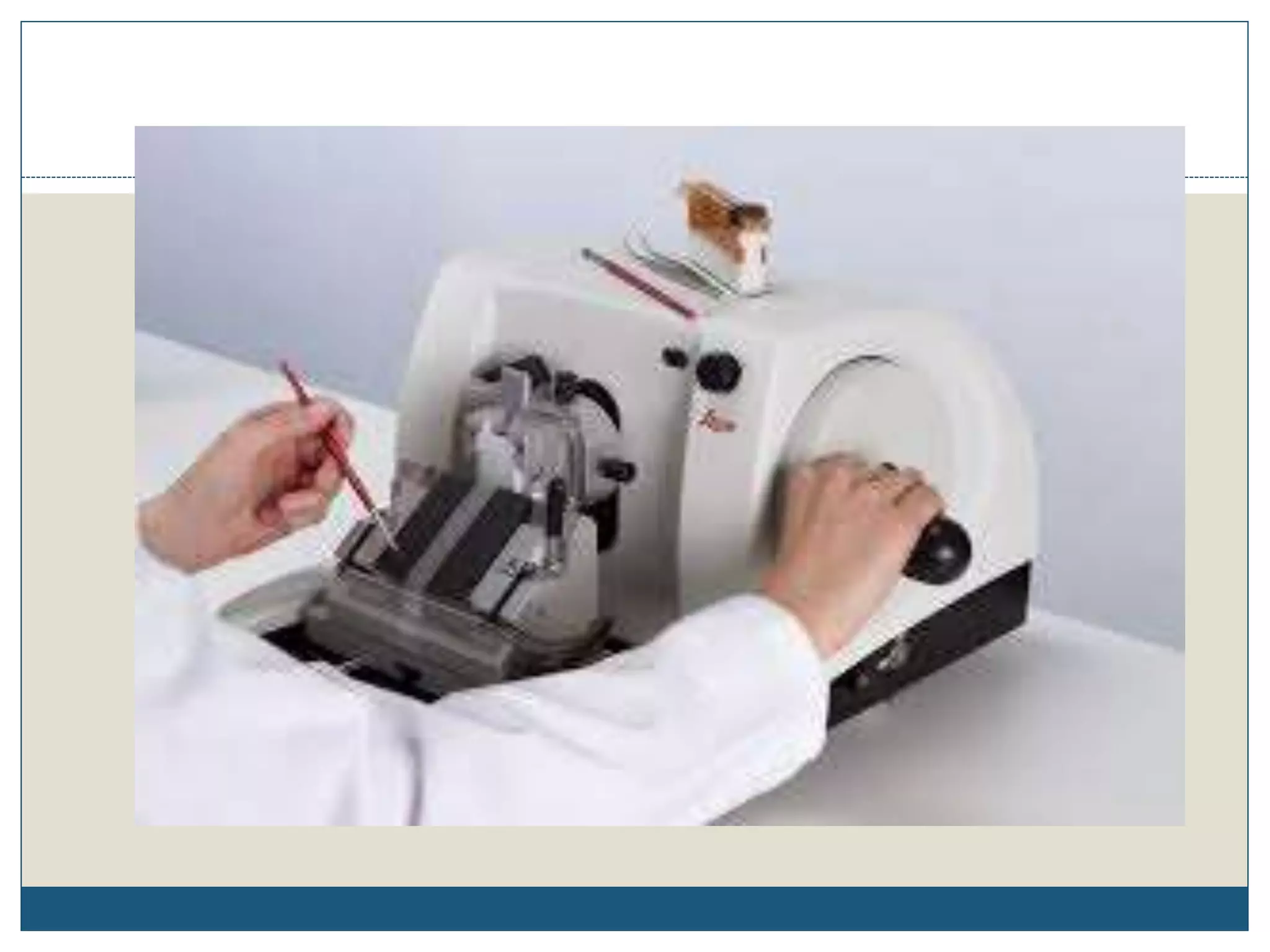
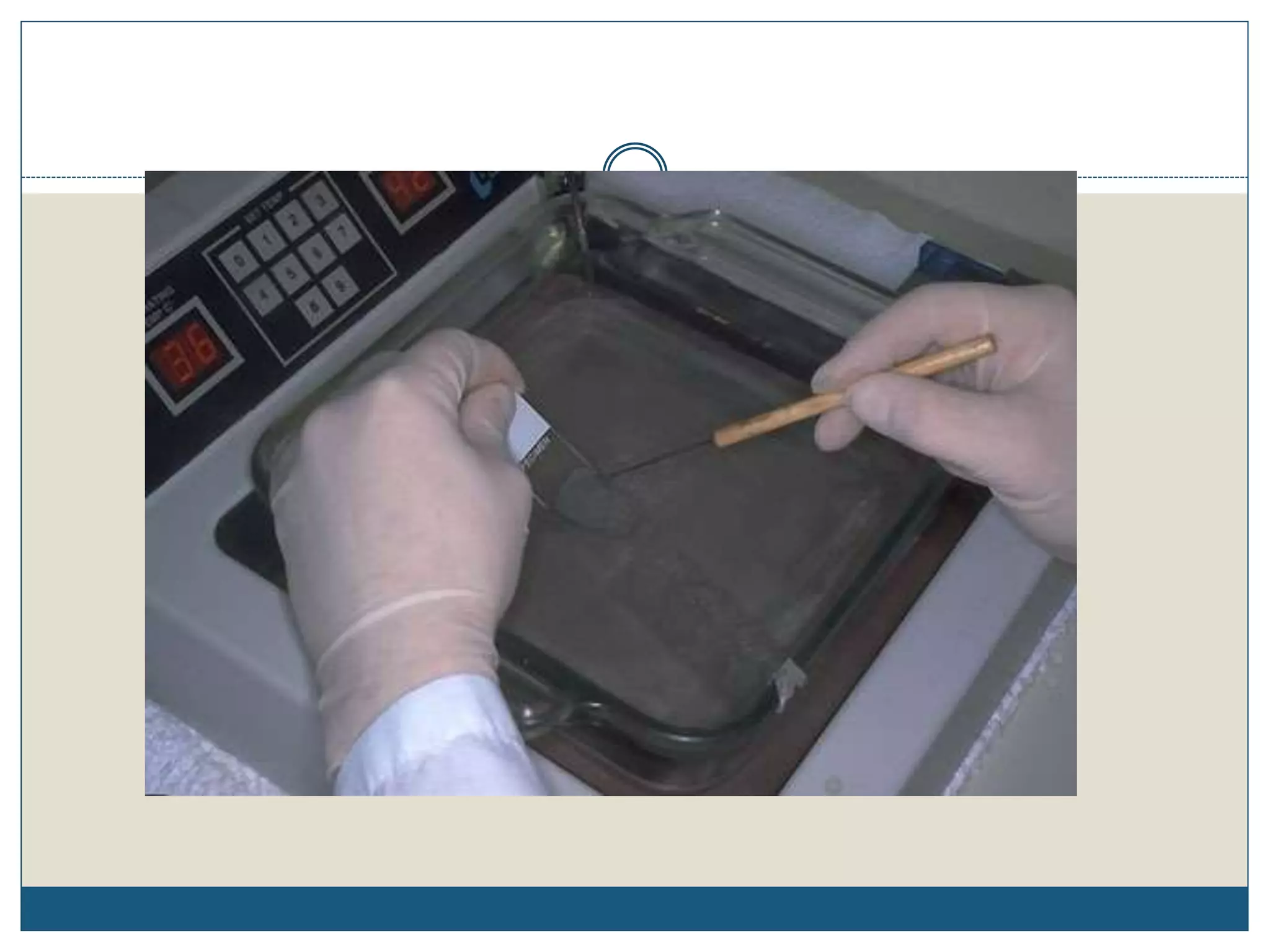
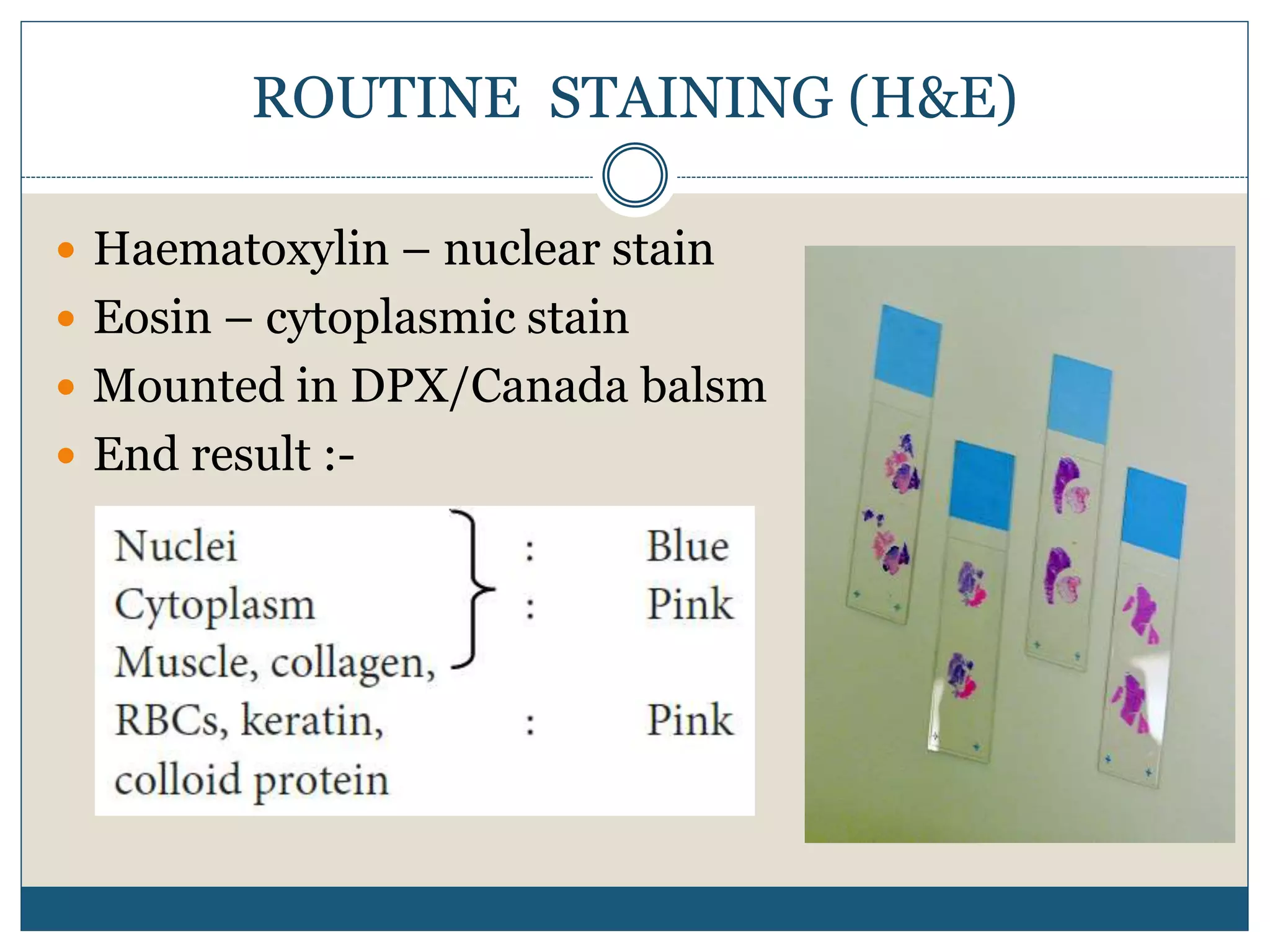
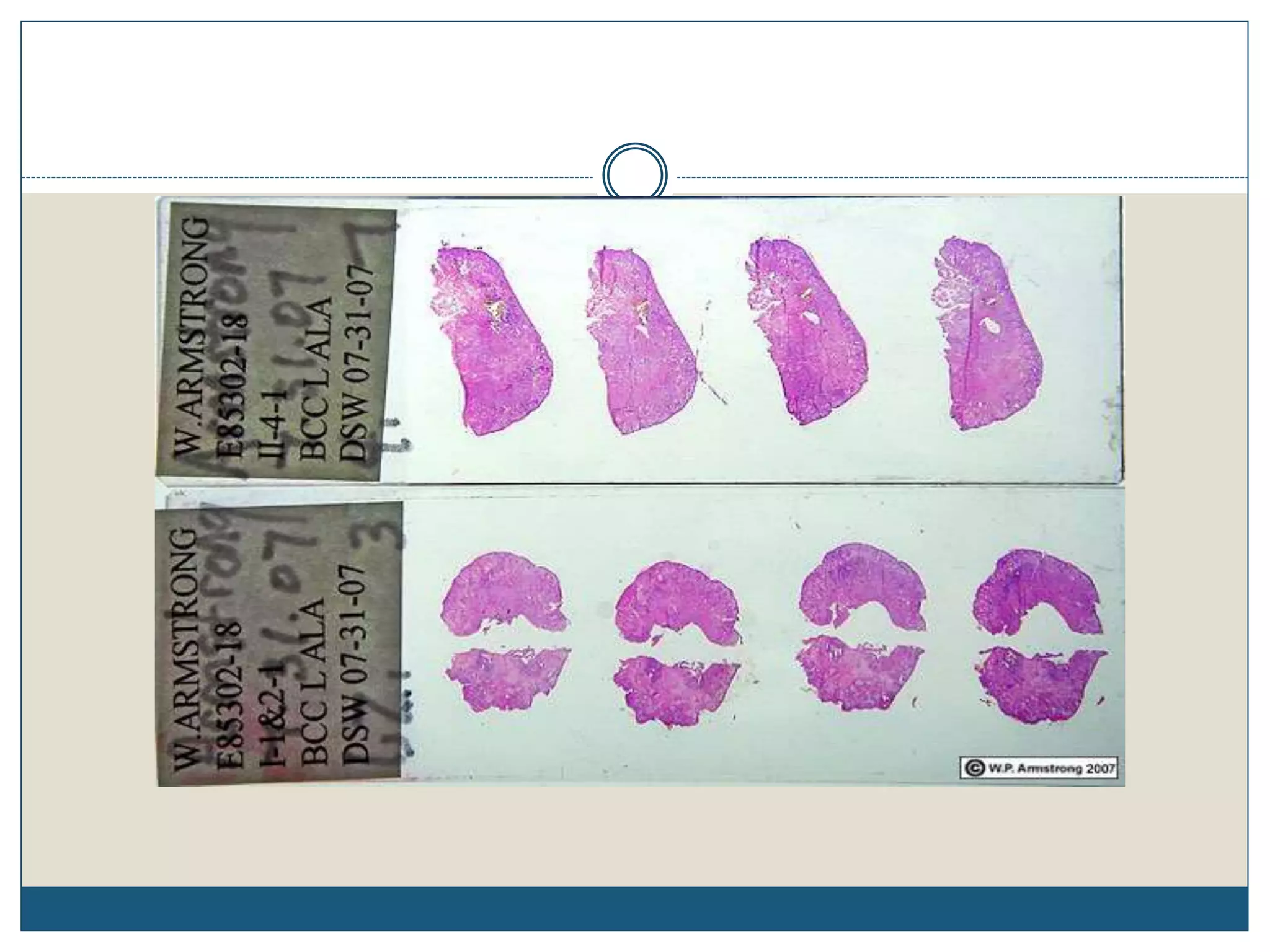
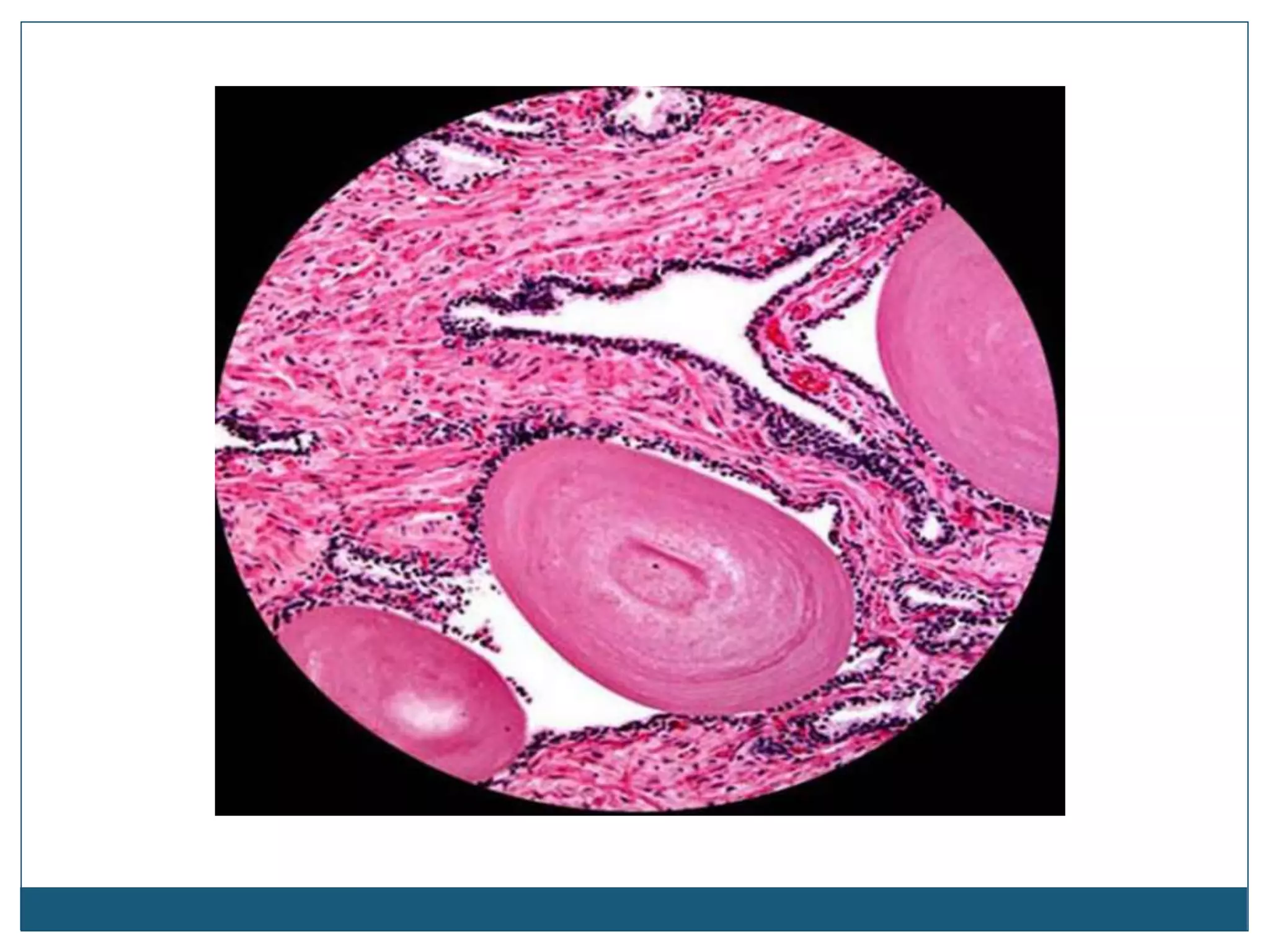
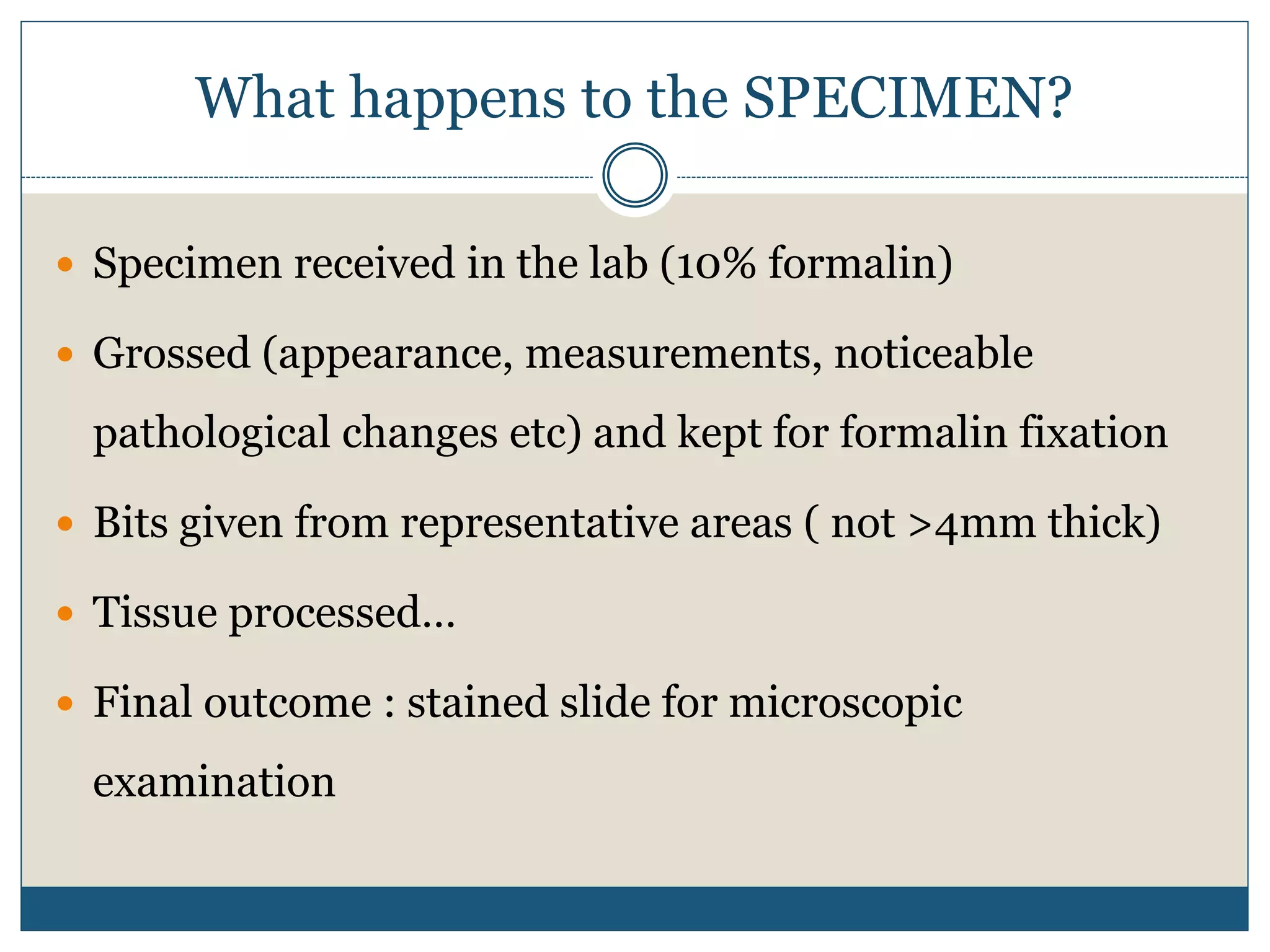
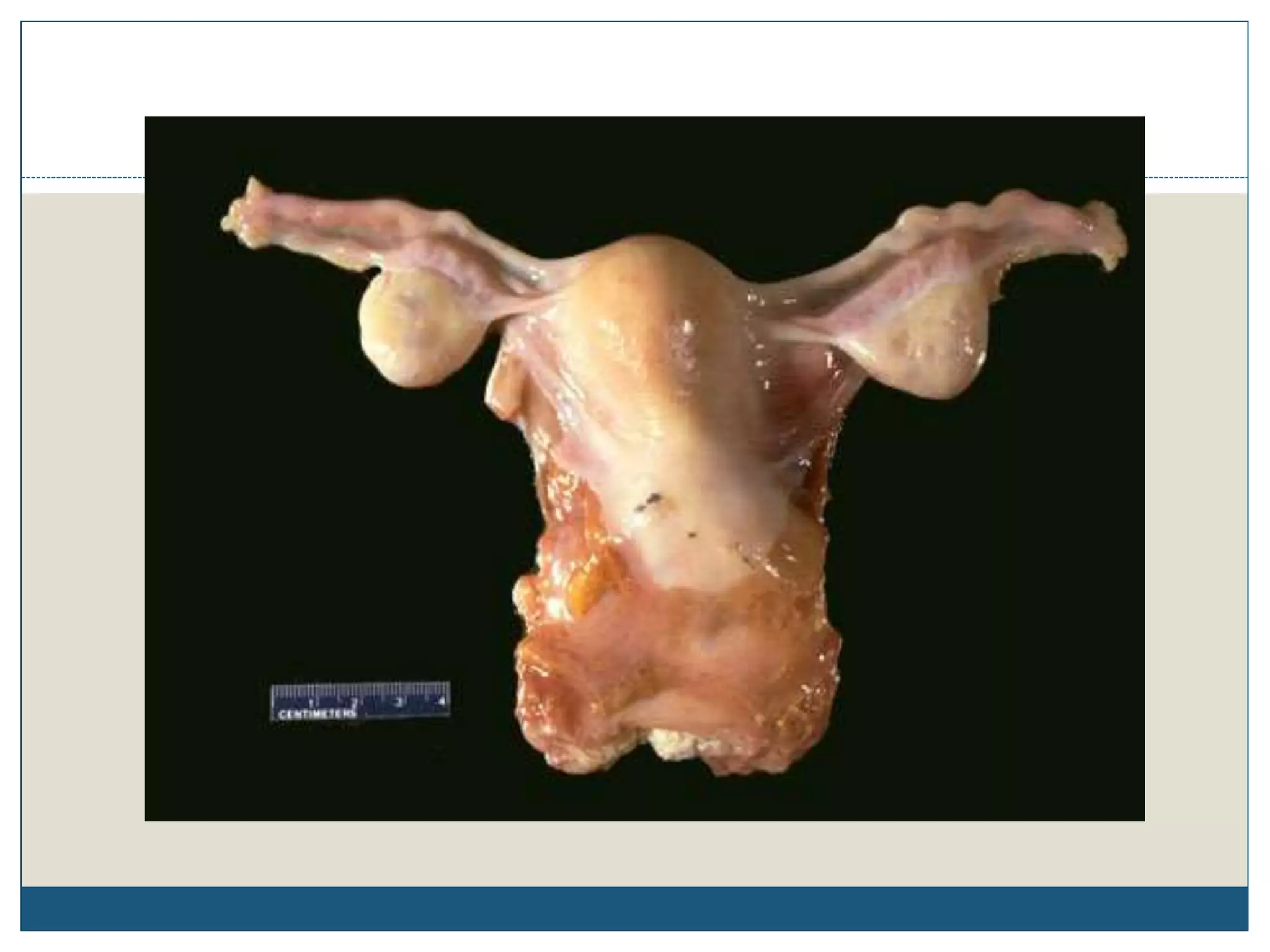
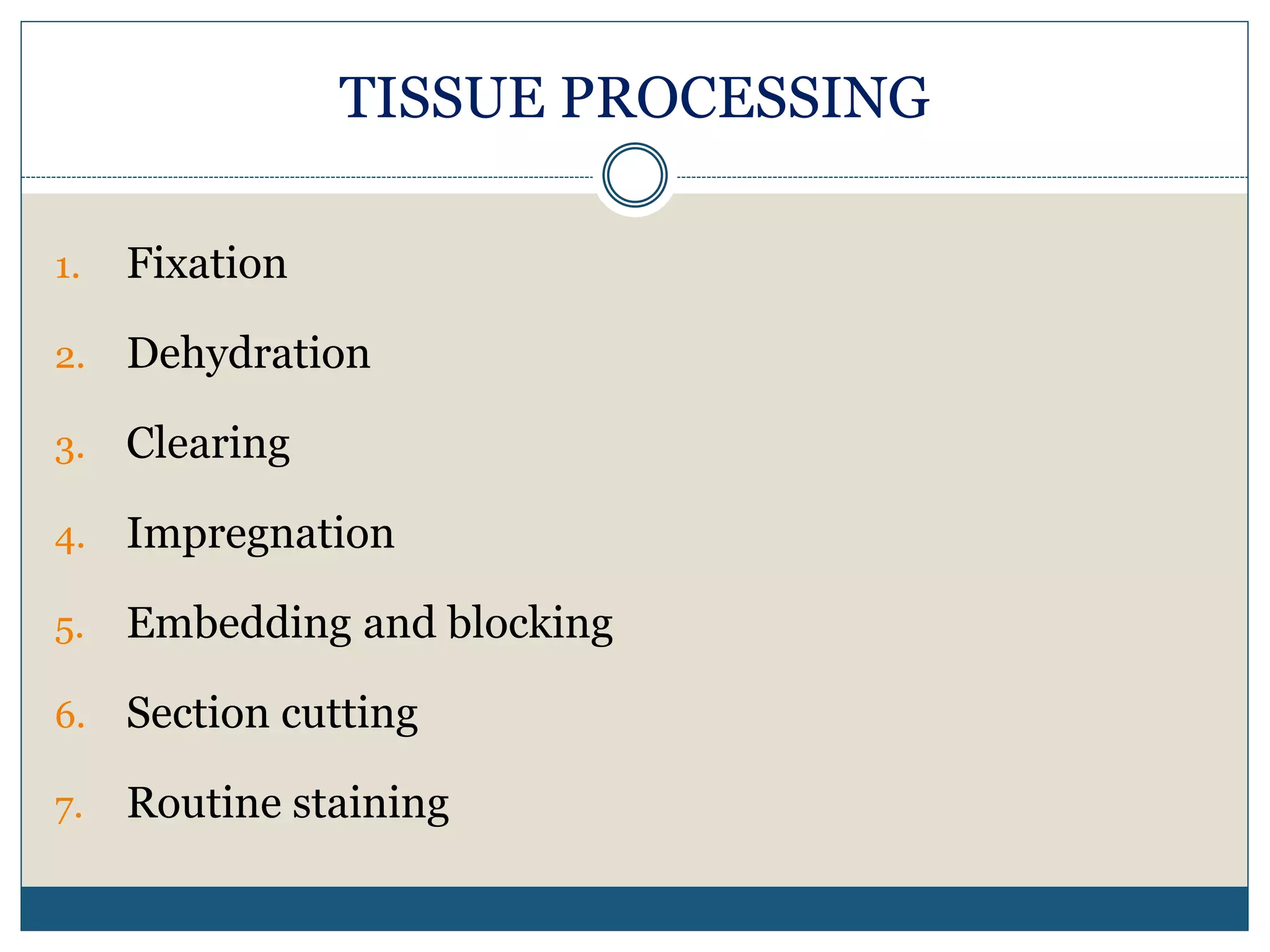
This document describes the process of tissue processing for microscopic examination. Tissue samples are first fixed in formalin to prevent decomposition. They are then dehydrated by passing through increasing concentrations of alcohol, cleared using xylene to remove the alcohol, and infiltrated with paraffin wax. The tissues are embedded in wax blocks and sectioned using a microtome. The sections are stained, most commonly with hematoxylin and eosin, which stain nuclei purple and cytoplasm pink. This allows examination under a microscope.





![TYPES OF FIXATIVES
[A]
Simple (one substance) Eg. Formalin
Compound (two or more) Eg. Bouin’s solution, Zencker’s
solution
[B]
Microanantomical – preserves anatomy
Cytological – cytoplasmic and nuclear features
Histochemical – constituents and enzymes](https://image.slidesharecdn.com/tissueprocessing-160906191122/75/Tissue-processing-12-2048.jpg)