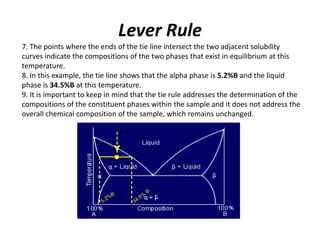
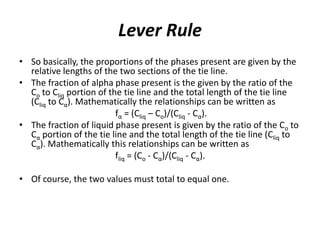
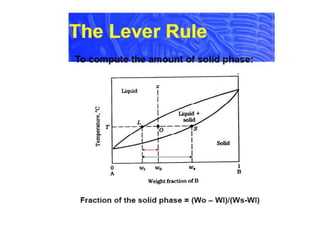
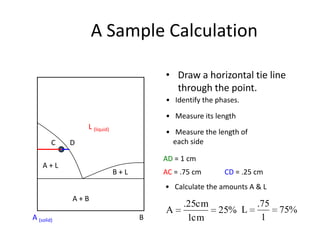
The document describes the lever rule, which is used to determine both the compositions of phases present at a given temperature in a multi-phase alloy system, as well as the relative amounts of each phase. It explains that a tie line is drawn through the given composition point on a phase diagram. The intercepts of the tie line with phase boundaries indicate the compositions of the phases. The relative amounts of each phase are inversely proportional to the distances from the composition point to the intercepts with each phase boundary.