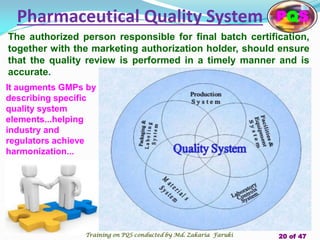
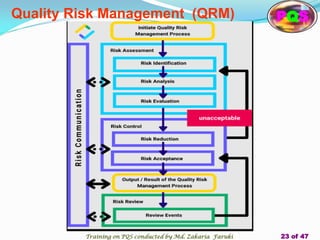
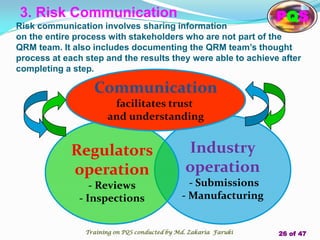
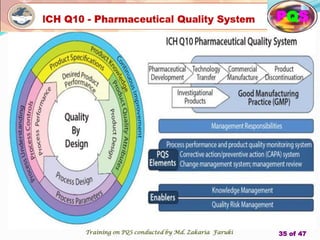
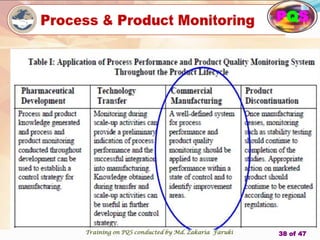
The document presents a comprehensive overview of the Pharmaceutical Quality System (PQS) and Quality Management System (QMS), outlining their significance in ensuring product quality, compliance, and customer satisfaction. It emphasizes the responsibilities of senior management, the role of quality risk management (QRM), and the necessity for continuous improvement across all stages of pharmaceutical production. Key elements such as risk assessment, control, communication, and periodic reviews are highlighted as essential practices for maintaining product quality and safety.