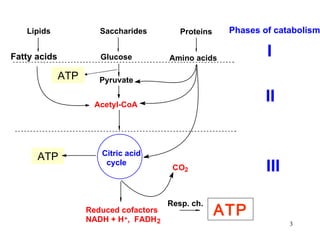
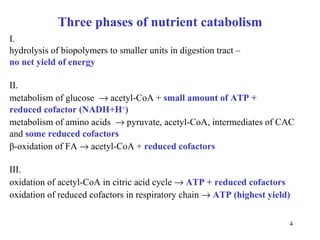
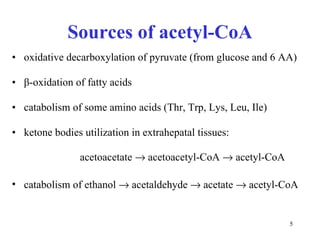
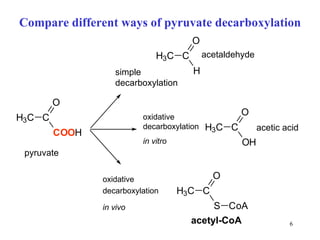
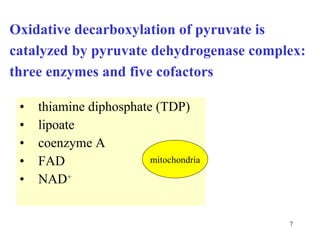
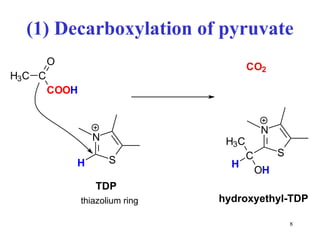
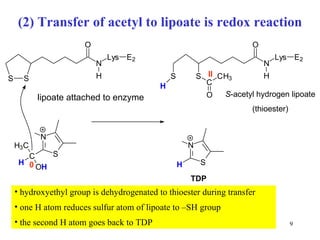
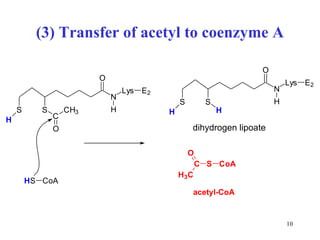
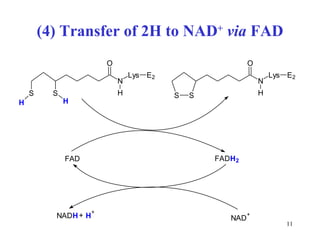
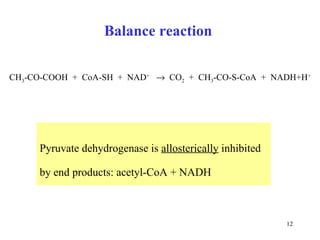
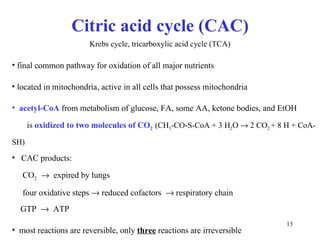
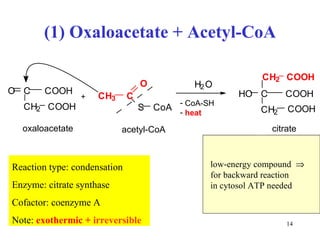
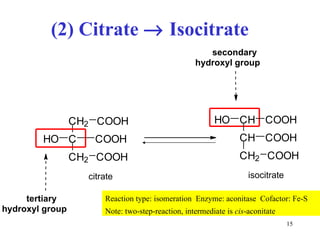
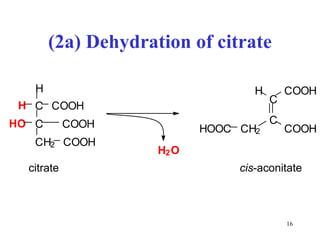
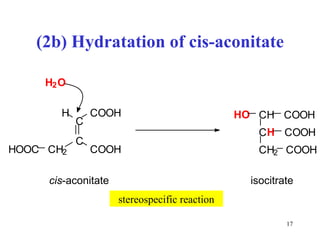
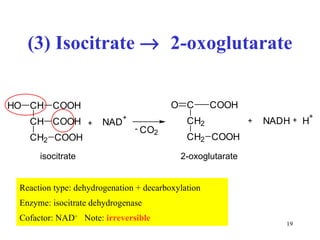
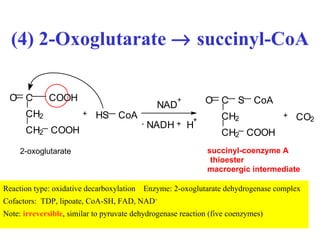
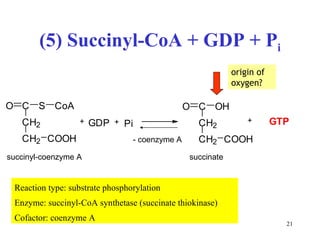
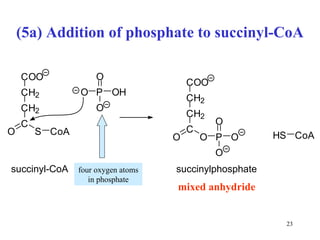
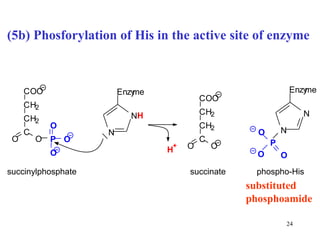
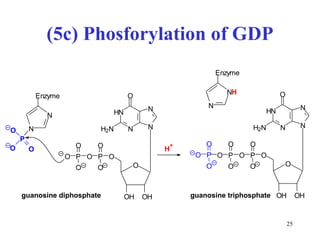
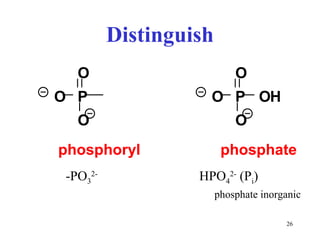
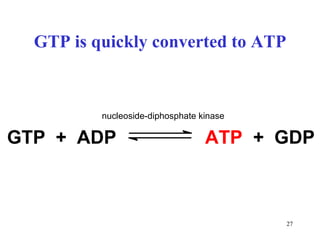
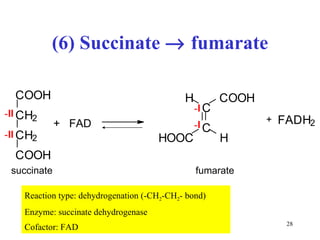
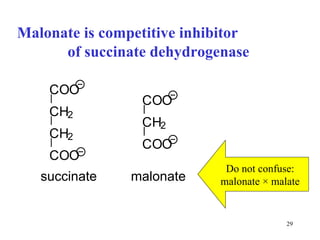
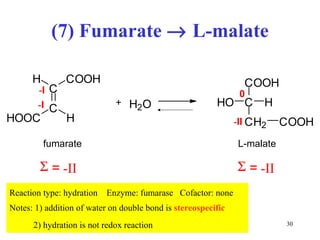
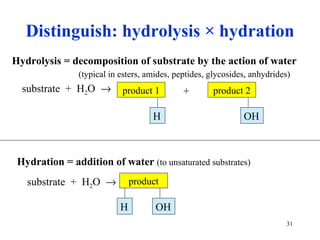
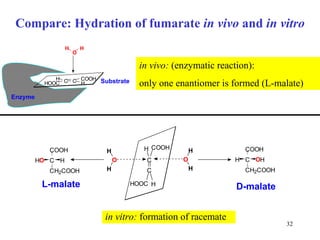
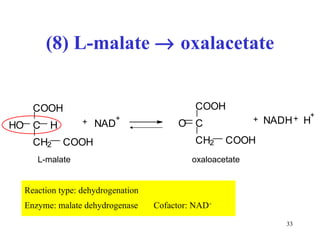
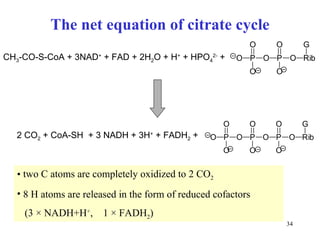
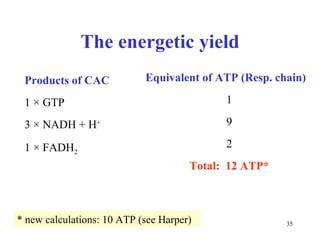
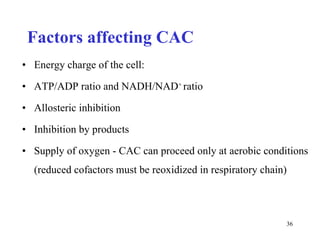
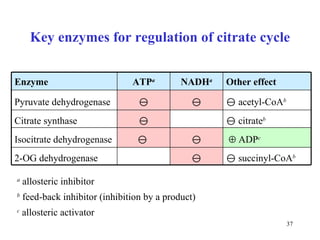
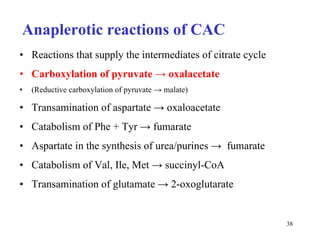
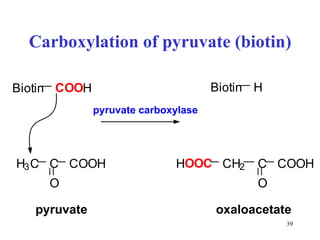
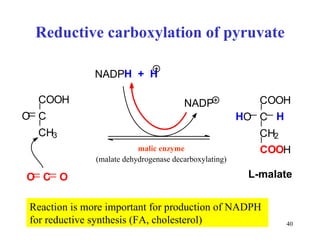
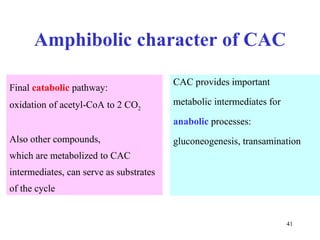
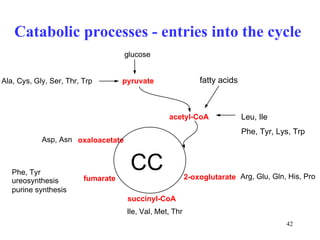
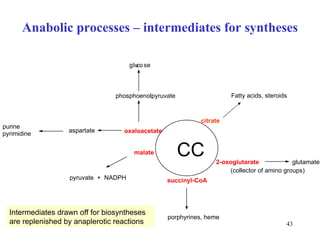
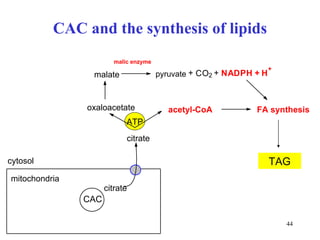
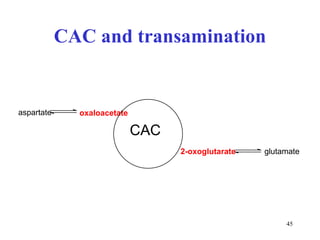
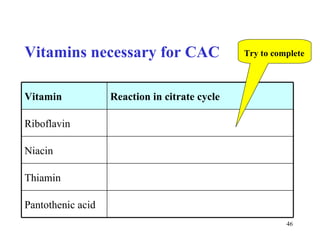
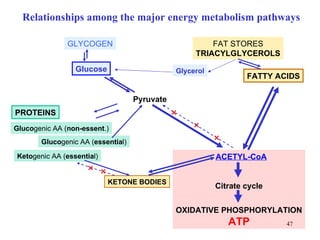
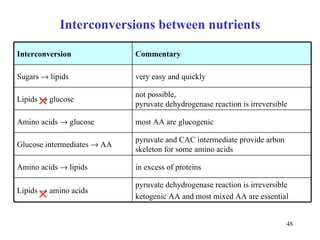
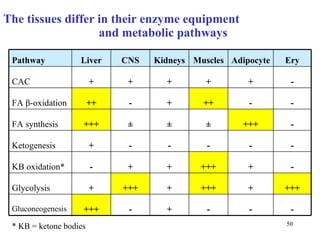
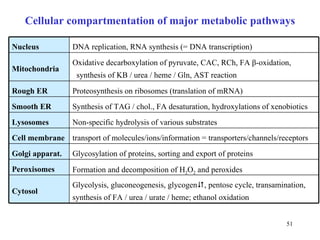
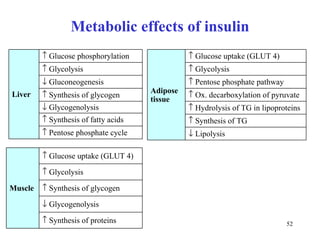
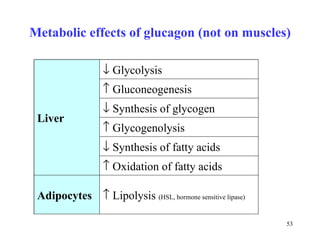
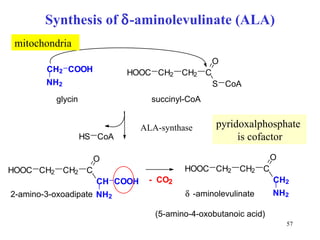
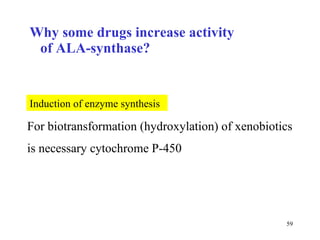
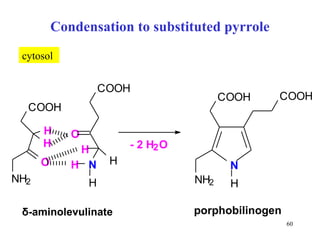
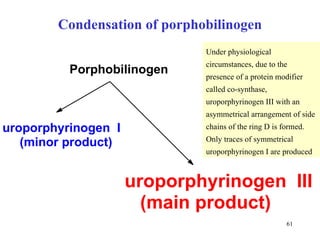
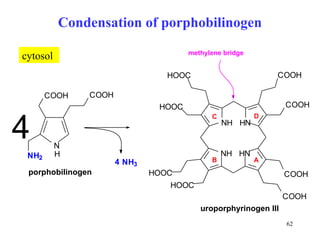
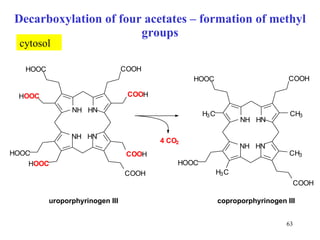
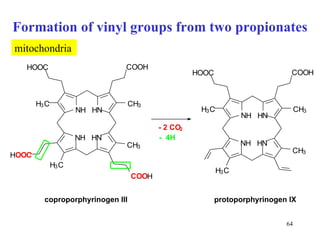
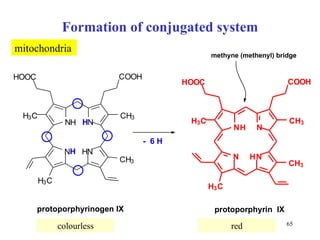
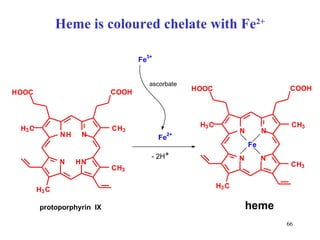
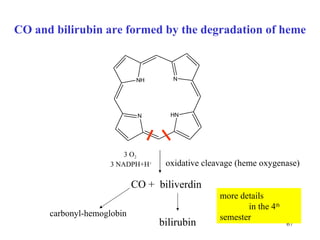
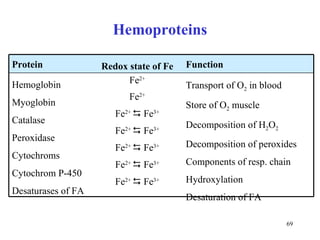
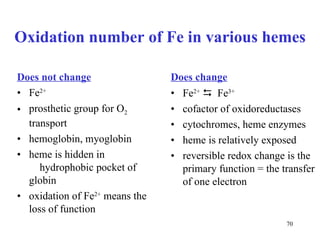
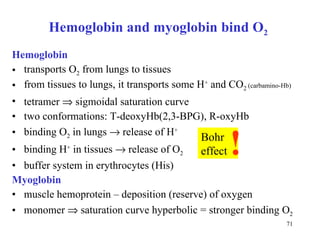
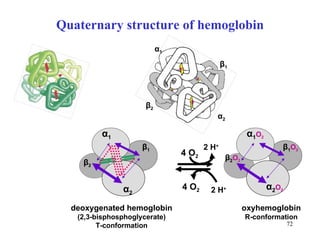
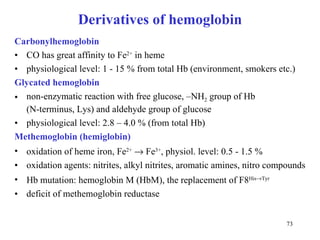
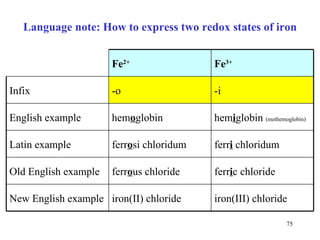
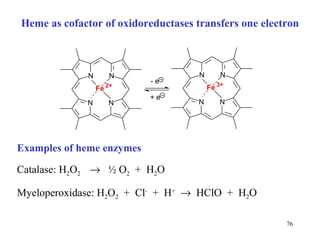
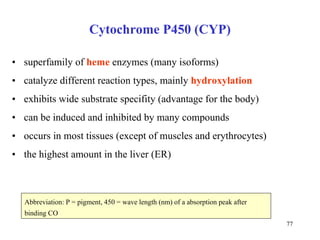
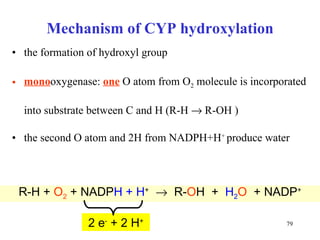
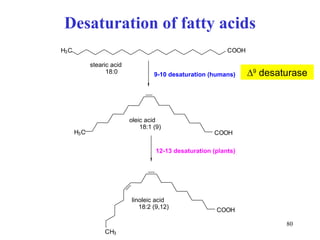
The citric acid cycle (CAC) is the final common pathway for the oxidation of nutrients. It occurs in the mitochondria of cells. Acetyl-CoA from various sources enters the CAC and is oxidized to CO2, producing reduced cofactors that drive ATP synthesis. The 8-step cycle produces ATP, GTP, and reduced cofactors NADH and FADH2. Key enzymes and cofactors regulate the cycle in response to energy demands and product inhibition. Anaplerotic reactions maintain CAC intermediate levels.