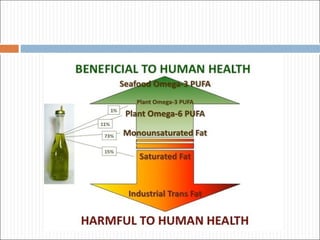
Polyunsaturated fatty acids (PUFAs) are important dietary and metabolic components. PUFAs can be synthesized in the body through a series of desaturation and elongation steps. Key PUFAs include omega-3 and omega-6 fatty acids. PUFAs play roles in membrane structure and function, energy metabolism, and act as precursors to bioactive lipid mediators like eicosanoids and docosanoids. PUFA intake is associated with cardiovascular and other health benefits.






































![CAT
Intermembrane
Space
OUTER
MITOCHONDRIAL
MEMBRANE
Cytoplasm
palmitoyl-CoA
AMP + PPi
ATP + CoA
palmitate
palmitoyl-CoA
Matrix
INNER
MITOCHONDRIAL
MEMBRANE
CPT-I
[2]
ACS
[1]
CPT-II](https://image.slidesharecdn.com/pufa2ppt26-150224223539-conversion-gate02/85/Pufa-2-ppt2-6-40-320.jpg)
![Figure 3 (bottom). Mitochondrial uptake via of palmitoyl-carnitine via
the carnitine-acylcarnitine translocase (CAT) (step 5 in Fig. 2).
Matrix
INNER
MITOCHONDRIAL
MEMBRANE
Intermembrane Space palmitoyl-carnitinecarnitine
CoApalmitoyl-CoA
CAT [3]
palmitoyl-carnitine
CPT-II
carnitine
CoApalmitoyl-CoA
[4]
CPT-I](https://image.slidesharecdn.com/pufa2ppt26-150224223539-conversion-gate02/85/Pufa-2-ppt2-6-41-320.jpg)
![CAT
Intermembrane
Space
OUTER
MITOCHONDRIAL
MEMBRANE
palmitoyl-carnitine
CoA
carnitine
Cytoplasm
palmitoyl-CoA
AMP + PPi
ATP + CoA
palmitate
palmitoyl-CoA
Matrix
INNER
MITOCHONDRIAL
MEMBRANE
[3]
palmitoyl-carnitinecarnitine
CoApalmitoyl-CoA
[4]
CPT-I
[2]
ACS
[1]
CPT-II](https://image.slidesharecdn.com/pufa2ppt26-150224223539-conversion-gate02/85/Pufa-2-ppt2-6-42-320.jpg)