5 phase rule and steels
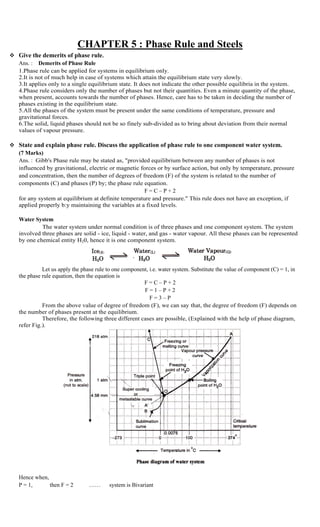
- 1. CHAPTER 5 : Phase Rule and Steels Give the demerits of phase rule. Ans. : Demerits of Phase Rule 1.Phase rule can be applied for systems in equilibrium only. 2.It is not of much help in case of systems which attain the equilibrium state very slowly. 3.It applies only to a single equilibrium state. It does not indicate the other possible equilibria in the system. 4.Phase rule considers only the number of phases but not their quantities. Even a minute quantity of the phase, when present, accounts towards the number of phases. Hence, care has to be taken in deciding the number of phases existing in the equilibrium state. 5.All the phases of the system must be present under the same conditions of temperature, pressure and gravitational forces. 6.The solid, liquid phases should not be so finely sub-divided as to bring about deviation from their normal values of vapour pressure. State and explain phase rule. Discuss the application of phase rule to one component water system. (7 Marks) Ans. : Gibb's Phase rule may be stated as, "provided equilibrium between any number of phases is not influenced by gravitational, electric or magnetic forces or by surface action, but only by temperature, pressure and concentration, then the number of degrees of freedom (F) of the system is related to the number of components (C) and phases (P) by; the phase rule equation. F = C – P + 2 for any system at equilibrium at definite temperature and pressure." This rule does not have an exception, if applied properly b:y maintaining the variables at a fixed levels. Water System The water system under normal condition is of three phases and one component system. The system involved three phases are solid - ice, liquid - water, and gas - water vapour. All these phases can be represented by one chemical entity H20, hence it is one component system. Let us apply the phase rule to one component, i.e. water system. Substitute the value of component (C) = 1, in the phase rule equation, then the equation is F = C – P + 2 F = 1 – P + 2 F = 3 – P From the above value of degree of freedom (F), we can say that, the degree of freedom (F) depends on the number of phases present at the equilibrium. Therefore, the following three different cases are possible, (Explained with the help of phase diagram, refer Fig.). Hence when, P = 1, then F = 2 …… system is Bivariant
- 2. P = 2, then F = 1 …… system is Monovariant P = 3, then F = 0 …… system is zero variant From the above equation it is clear that, for arty one component system, the maximum number of degree of freedom is two and most convenient variables are pressure and temperature. In the above phase diagram of water system following salient features are observed: 1. The curves OA, OB and OC. 2. The areas AOC, AOB and BOC. 3. 3. The triple point '0' and 4. The metastable curve. (OA/ ) 1. The curves OA, OB and OC These three curves meet at the point '0' (called as tripple point) and divide the diagram in to three areas. Therefore, these three curves ar1 known as boundary lines. Curve OA (Vapour Pressure Curve) The curve OA terminates at A, the critical point 218 atm. and 3740 temperature. It represents the vapour pressure of liquid water at different temperatures. The two phases water and water vapour coexist in equilibrium along this curve. Here, are two phases (P = 2) and one component (C = 1), therefore F = 1 – 2 + 2 = 1 Hence, system is monovariant or univariant or having one degree of freedom. When the vapour pressure is equal to one atmosphere, the corresponding temperature C as shown in figure is the boiling point of water, i.e. 100 C Curve OB (Sublimation curve) The curve OB terminates at B, the absolute zero, Le. - 2730 temperature. It shows the vapour pressure of solid ice at different temperature The two phases solid-ice and water-vapour coexist in equilibrium along this curve. Therefore, degree of freedom for this system is also one and system is monovarient. Curve OC (Fusion curve) The curve OC terminates at C, the critical pressure. The two phases solid-ice and liquid-water coexist in equilibrium. This curve indicates that the melting point of ice decreases with increase of pressure. The one atmosphere (1.0 atm.) line meets the fusion (freezing/melting) curve at O°C which is the normal melting point of ice. Again, along the curve OC, there are two phases in equilibrium and system is of one component. Therefore, the system is monovarient. From the above discussion, we can say that, along the curves OA, OB and OC there are two phases in equilibrium and one component. Therefore, F = C – P + 2 F = 1 – 2 + 2 F = 1 Hence, each two phases system has one degree of freedom, i.e. system is univarient or monovarient. 2. The areas AOC, AOB and BOC The regions or areas between the curves show the conditions of temperature and pressure under which a single phase, i.e. ice, water or water vapour is capable of stable existence. Thus 1. Area AOC represents conditions for liquid phase, i.e. water. 2. Area AOB represents conditions for gaseous phase, i.e. water vapour. 3. Area BOC represents conditions for solid phase, i.e. ice. In all the three areas, there being 'one phase' and 'one component'. Therefore, F = C – P + 2 F = 1 – 1 + 2 F = 2 Hence, each system has two degree of freedom, i.e. system is bivarient or divarient. 3. Triple point All the three curves, OA, OB and OC meet at the point 0 called as tripple point, where all the three phases solid, liquid and vapour are simultaneously in equilibrium. This triple point occurs at O.0075°C and 4.58 mm Hg pressure. Since, there are three phases and one component, therefore
- 3. F = C – P + 2 F = 1 – 3 + 2 F = 0 The system at tripple point is zero variant or nonvariant. Thus, neither pressure nor temperature can be altered. Even slightly changed three phases would not exist if one of the phase disappears. 4. Metastable curve (curve OA’) This curve is also known as supercooling (water/vapour) curve. This is the extension of curve OA, i.e. vapour pressure curve. That is water can be supercooled by eliminating solid particles carefully which includes crystallization. The supercooled water system is unstable, i.e. metastable. It at once reverts to the stable system ice or vapour on the slightest disturbance. The metastable vapour pressure of super cooled water is higher than vapour pressure of ice. What are alloys steels? What are the effects of following alloying elements on alloy steels : (i) Nickel (ii) Chromium (iii) Cobalt (iv) Tungsten Ans. : Metals possess many useful properties, such as high malleability, ductility, luster, good electrical conductivity being a few to mention. But, in nature metals are not available in pure state. When metals are extracted from their natural sources - i.e. minerals or ores, some impurities are carried along with the pure metals. Hence, to get pure metal, further purification has to be done by various different methods. But after all this processing, the pure metal obtained from its ore, loses some vital characteristics and becomes practically useless for engineering purposes. Some of such characteristics are, its tensile strength, corrosion resistance and hardness. The pure metals are very soft, highly chemically reactive, highly malleable and ductile. Thus changes in these vital properties, reduce shock and wear resistance of metals. The high chemical reactivity makes pure metal susceptible to corrosion. The properties of pure metals can be improved by alloying the pure metal with another suitable meta/non-metal, e.g. iron in pure state can be alloyed to get steel, which shows the desired properties such as hardness, toughness, high corrosion resistance etc. Here, steel is an alloy of iron with carbon (non-metal), chromium/manganese (metals) etc. An alloy is a solid mixture of two or more metals or non-metals. Alloy must have necessarily, (i) at least one metal (base metal) (ii) at least one additional metal or non-metal. Element Special effects ChromiumEnhance hardenability, corrosion and oxidation resistance, increase high temperature strength. In high carbon steels, it increases abrasion resistance. Cobalt Contributes to hardness of steel. Nickel Along with other elements, renders moderate to high hardenability ; enhance strength of unhardened steels by solid solution effect ; enhances toughness in pearlitic – ferritic steels. Tungsten It helps to form hard and abrasion resisting carbide film in tool steels. Imparts high temperature hardness in tempered steels. It enhances creep strength in some high temperature steels. What is triple point in phase diagram? Explain it with reference to one component water system phase diagram.
- 4. All the three curves, OA, OB and OC meet at the point 0 called as tripple point, where all the three phases solid, liquid and vapour are simultaneously in equilibrium. This triple point occurs at 0.0075°C and 4.58 mm Hg pressure. Since, there are three phases and one component, therefore F = C – P + 2 F = 1 – 3 + 2 F = 0 The system at tripple point is zero variant or nonvariant. Thus, neither pressure nor temperature can be altered. Even slightly changed three phases would not exist if one of the phase disappears. Explain any two of the following terms : 1. Phase 2. Components 3. Degrees of freedom Ans. : (1) Phase A phase is defined as any homogeneous, physically distinct and mechanically separable portion of a system, which is separated from other such parts of the system by definite boundary surfaces. Examples 1. In water. system, at freezing point of water, an equilibrium exists where ice, water and water vapours are the three phases, each of which is physically distinct and homogeneous, and with definite boundaries between ice, water and water vapours, as, 2. All gases mix freely to form homogeneous mixtures. Therefore, any mixture of gases, say and and forms one phase only. 3. Two completely miscible liquids yield an uniform solution. Thus, a solution of alcohol and water is a one phase system. (2) Components Definition The term component is defined as, "the smallest number of independently variable constituents taking part in the state of equilibrium by means of which the composition of each phase can be expressed directly or in the form of chemical equation". Examples 1. In water system, we have three phases, i.e. ice (Solid), water (Liquid) and water vapour (Gaseous) in equilibrium. Each of these phases are different physical forms of the same chemical substance, i.e. H20. Hence, system is regarded one component system. 2. In sulphur system, there are four phases, Le. rhombic sulphur, monoclinic sulphur, liquid sulphur and sulphur
- 5. vapour. The composition of all four phases can be expressed by one chemical individual sulphur (S). Hence, sulphur system is regarded as one component system. 3. When calcium carbonate is heated in a closed vessel, the following reaction takes place. Degree of Freedom (Variance) Definition Term degree of freedom is defined as, "the minimum number of independently variable factors such as temperature, pressure and composition of the phases which must be arbitrarily specified in order to represent perfectly the condition of a system". Examples In case of water system : (a) If all' the three phases are in equilibrium, then no condition need to be specified because the three phases can be in equilibrium only at particular temperature and pressure, The system is no degree of freedom or invariant or zero variant or non-variant. (b) If condition like temperature or pressure in altered, three phases will not remain in equilibrium and one of the phase disappears. For the following system : We must state either the temperature or pressure to define it completely. Hence, the degree of freedom is one or system univariant. , (c) For a system consisting of water in vapour phase only we must state the values of both, the temperature and pressure in order to 'describe the system completely. Hence, the system has two degree of freedom or system is bivariant. State and explain condensed phase rule. (3 Marks) Ans. : Condensed or Reduced Phase Rule When a single phase is present in a two component system, then the degree of freedom (F) is represented by following equations; F = C – P + 2 F = 2 – 1 + 2 F = 3 From the values of F (F = 3) we can say that, three variables must be specified in order to describe the condition of phase, i.e. in addition to temperature and pressure the concentration of one of the component has to be given. What are plain carbon steels? How can they be classified on the basis of carbon contents? (3 Marks) Ans. : The alloys of iron with other metal (s) or/and non-metal are known as ferrous alloys. These are commonly known as alloy steels. The metal iron generally forms alloys by mixing with carbon, and any other element (metal) such as either nickel alone or nickel and chromium both. Based on this, the alloy of iron and carbon (i.e. steels - widely known as plain carbon steels) are either, (a) Three components i.e. (Fe, C, Ni) or (b) Four components i.e. (Fe, C, Ni, Cr) Since these 'steels essentially contain iron and carbon, are known as plain carbon steels. The percentage of carbon in steels ranges from 0.008% to 2%. The plain carbon steels are further classified/named on the basis of its carbon content as,
