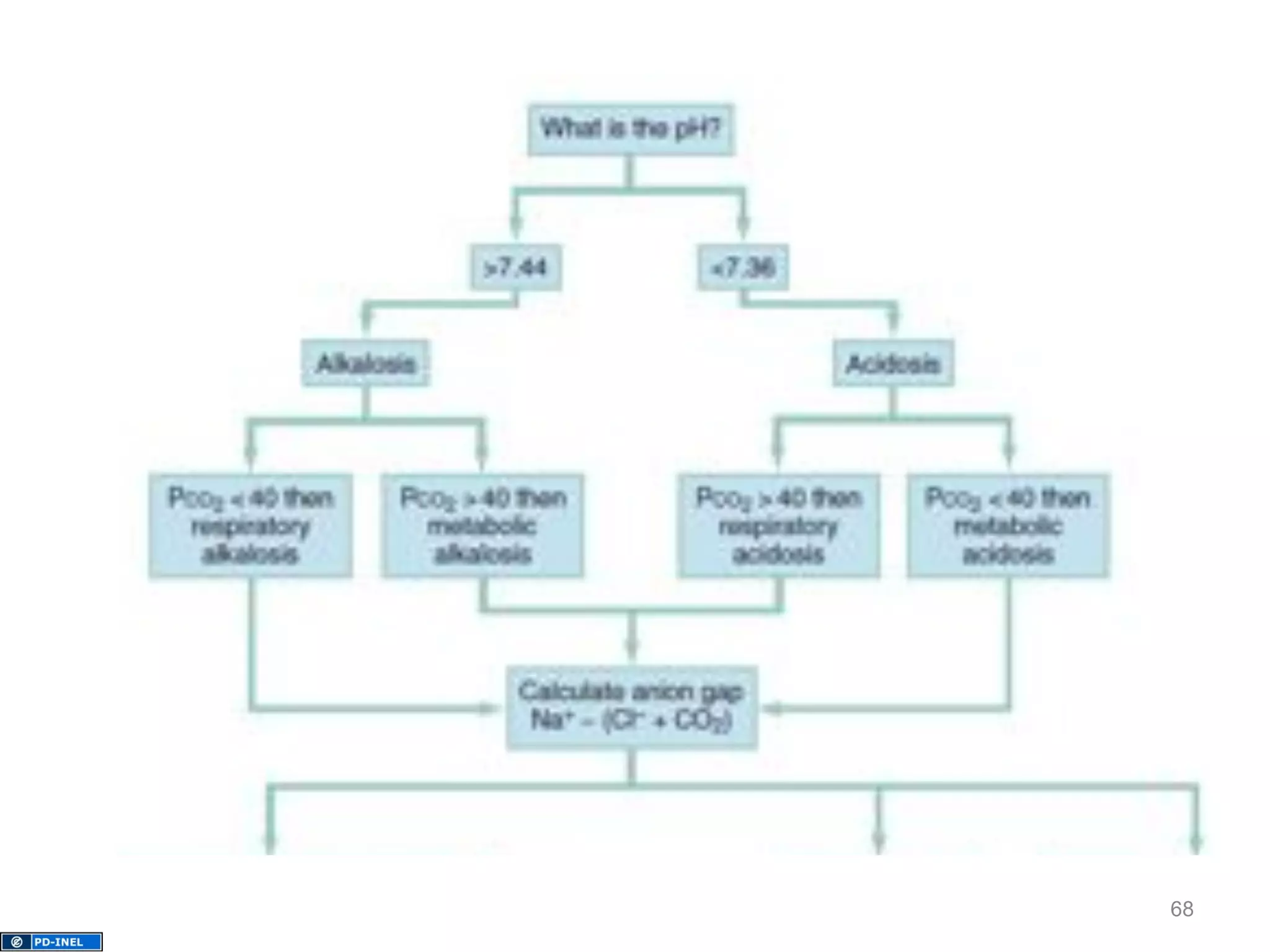
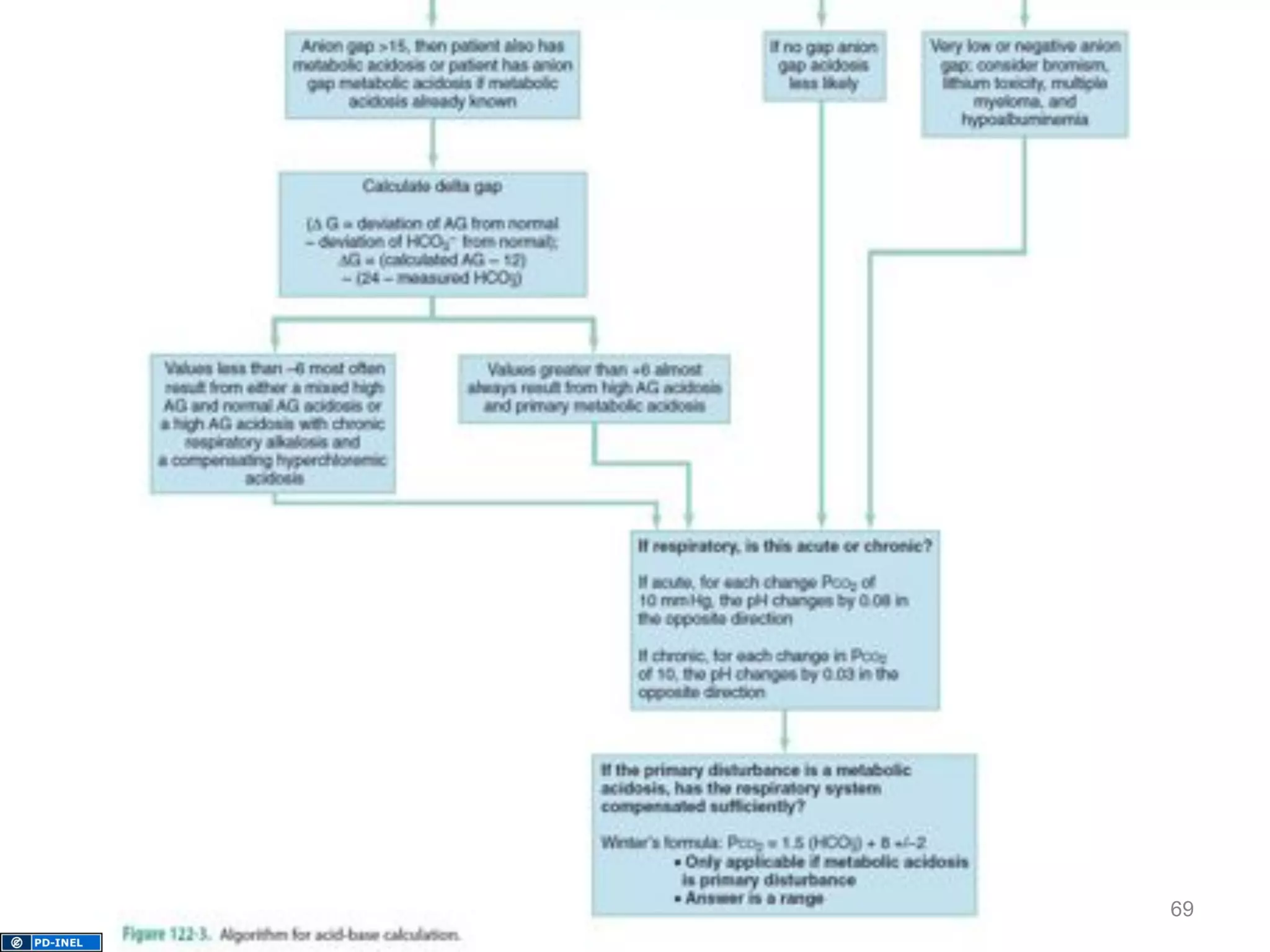
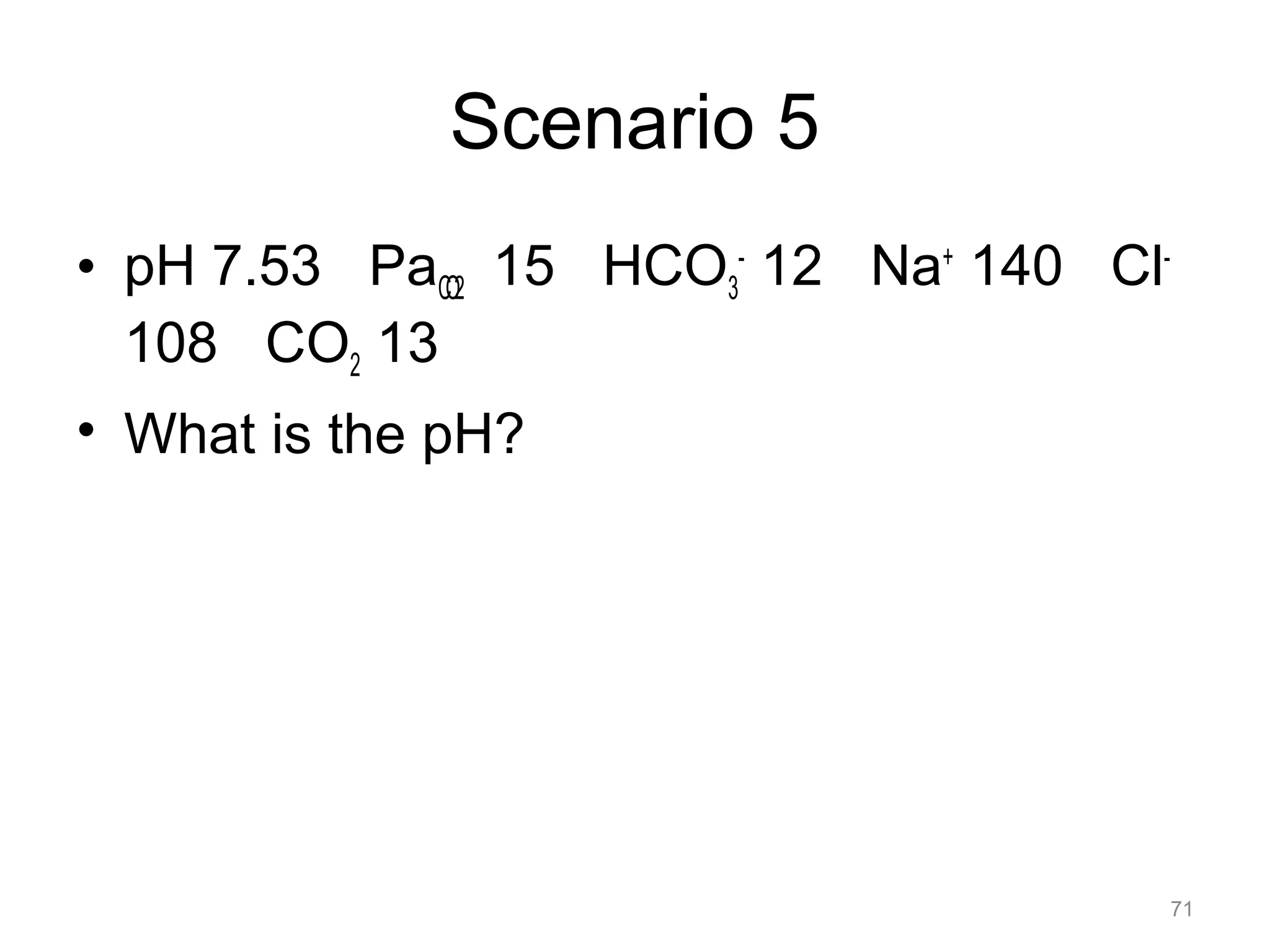
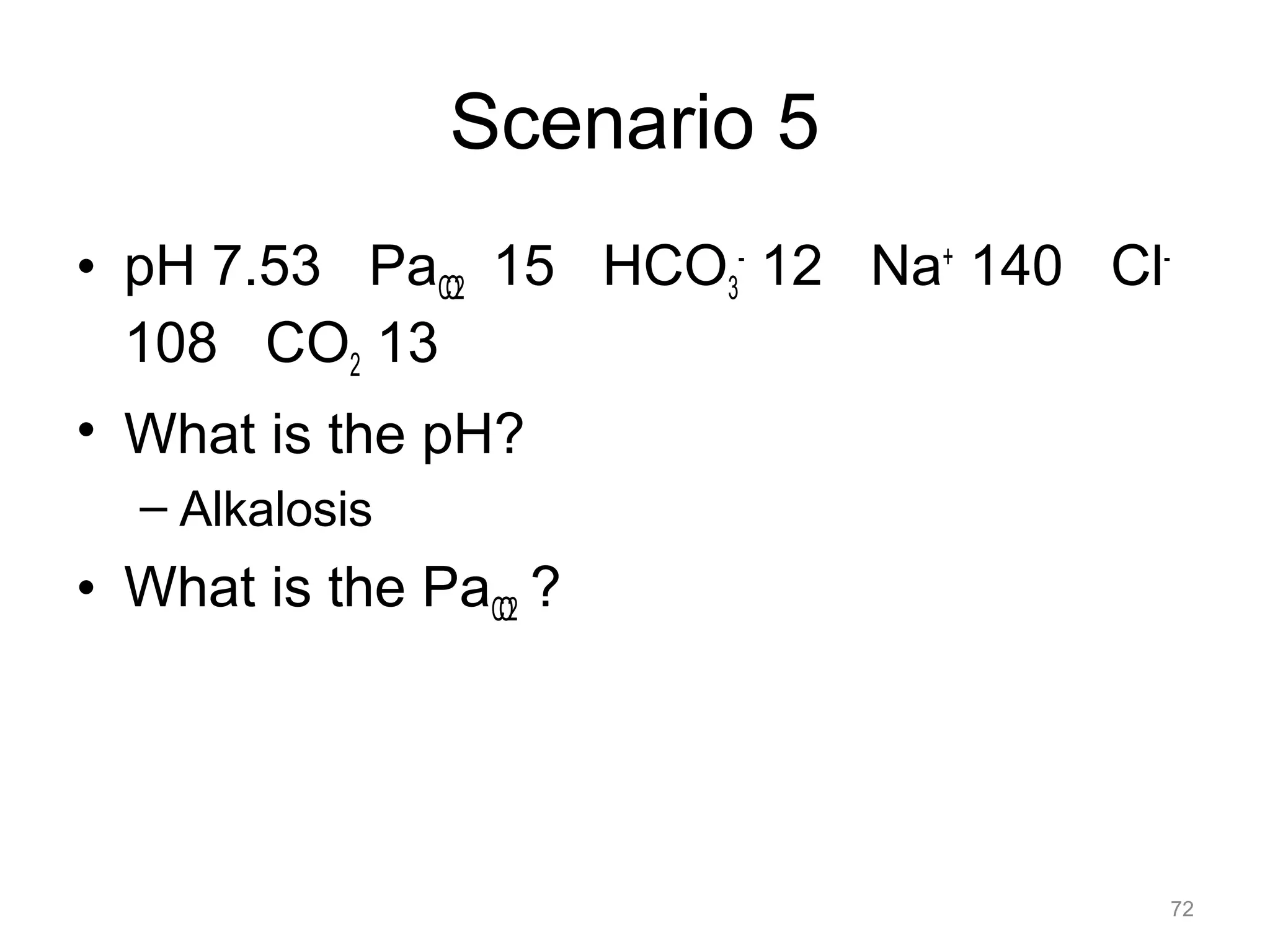
The document discusses acid-base disorders, outlining the importance of maintaining acid-base balance and the physiological mechanisms involved, including the roles of the kidneys and lungs. It covers classification, diagnostic criteria, and examples of conditions leading to respiratory and metabolic acidosis and alkalosis. Treatment strategies emphasize addressing underlying causes to normalize pH levels.




![Principles of Acid-Base
Disorders
• Kidneys, Lungs and Buffers maintain
serum pH between 7.36 and 7.44
• Blood pH is determined by the ratio of
serum bicarbonate concentration ([HCO3-])
and partial pressure of CO2 (PaCO2 )
5](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-5-2048.jpg)
![Principles of Acid-Base
Disorders
• Metabolic acid-base disorders and
secondary metabolic compensation alter
[HCO3-]
• Respiratory acid-base disorders and
secondary respiratory compensation alter
(PaCO2 )
6](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-6-2048.jpg)
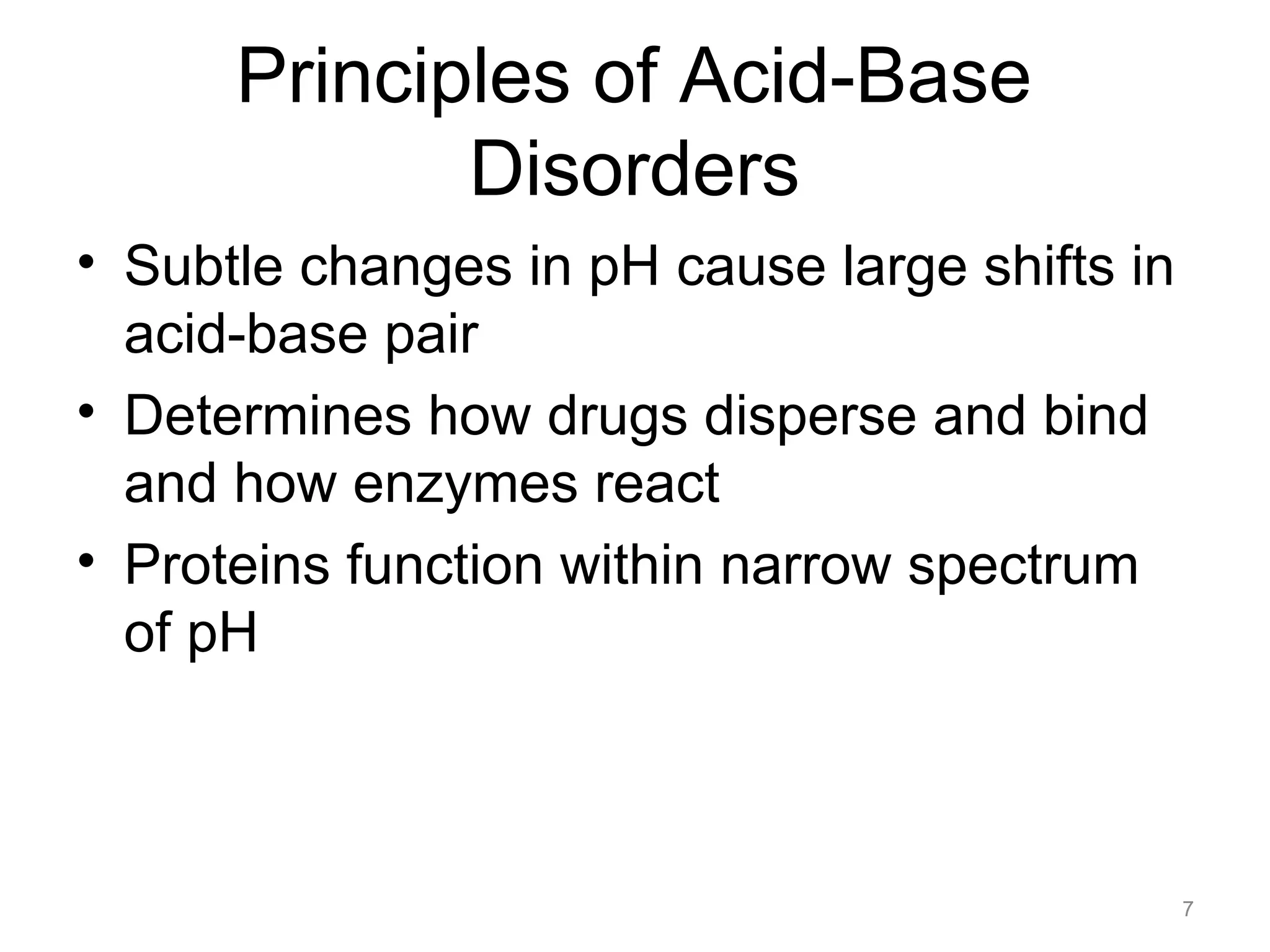
![Principles of Acid-Base
Disorders
• Acidemia: serum pH < 7.36
• Alkalemia: serum pH > 7.44
• Acidosis: pathologic process that lowers
[HCO3-] or raises PaCO2
• Alkalosis: pathologic process that raises
[HCO3-] or lowers PaCO2
8](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-8-2048.jpg)
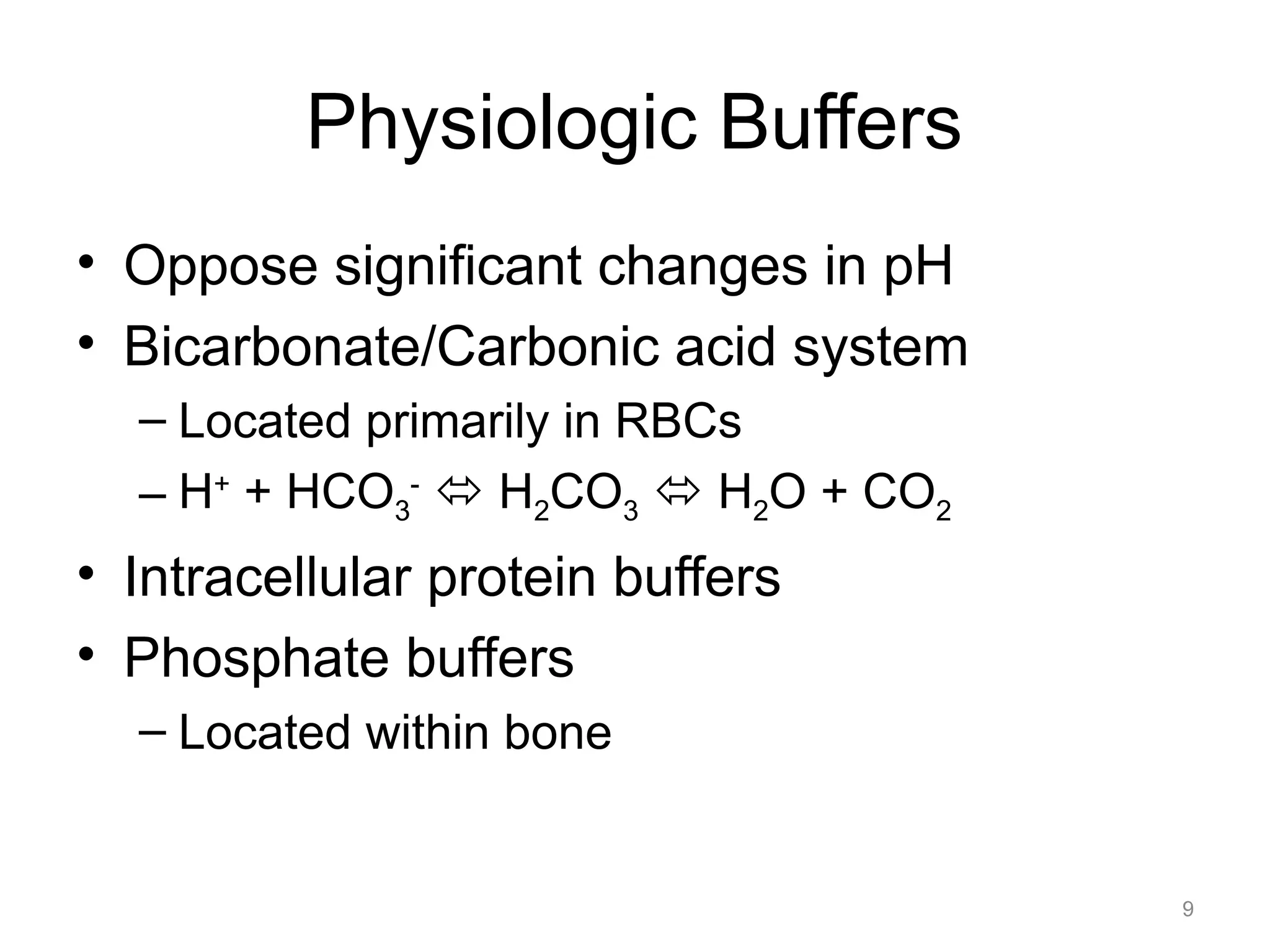
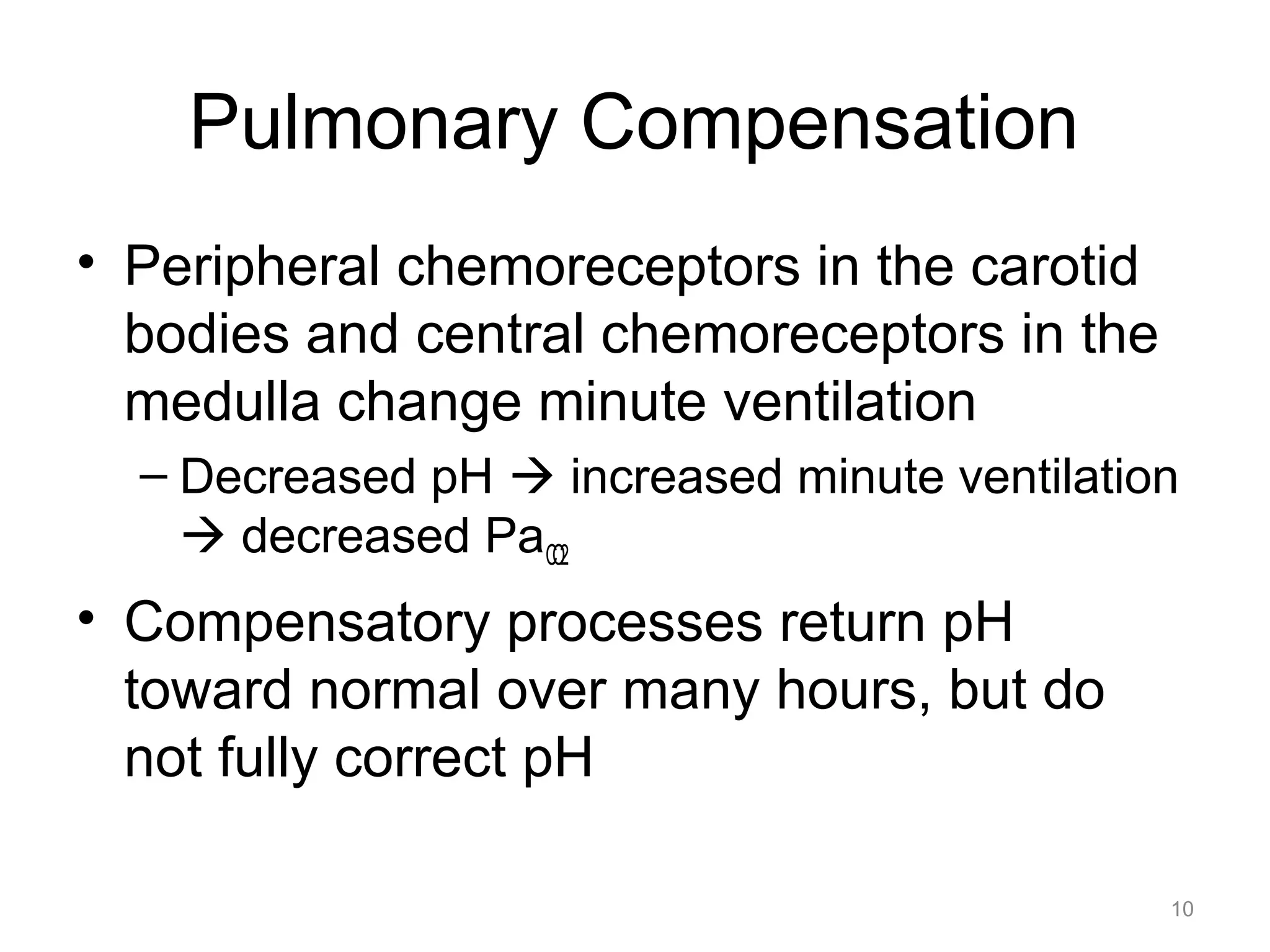
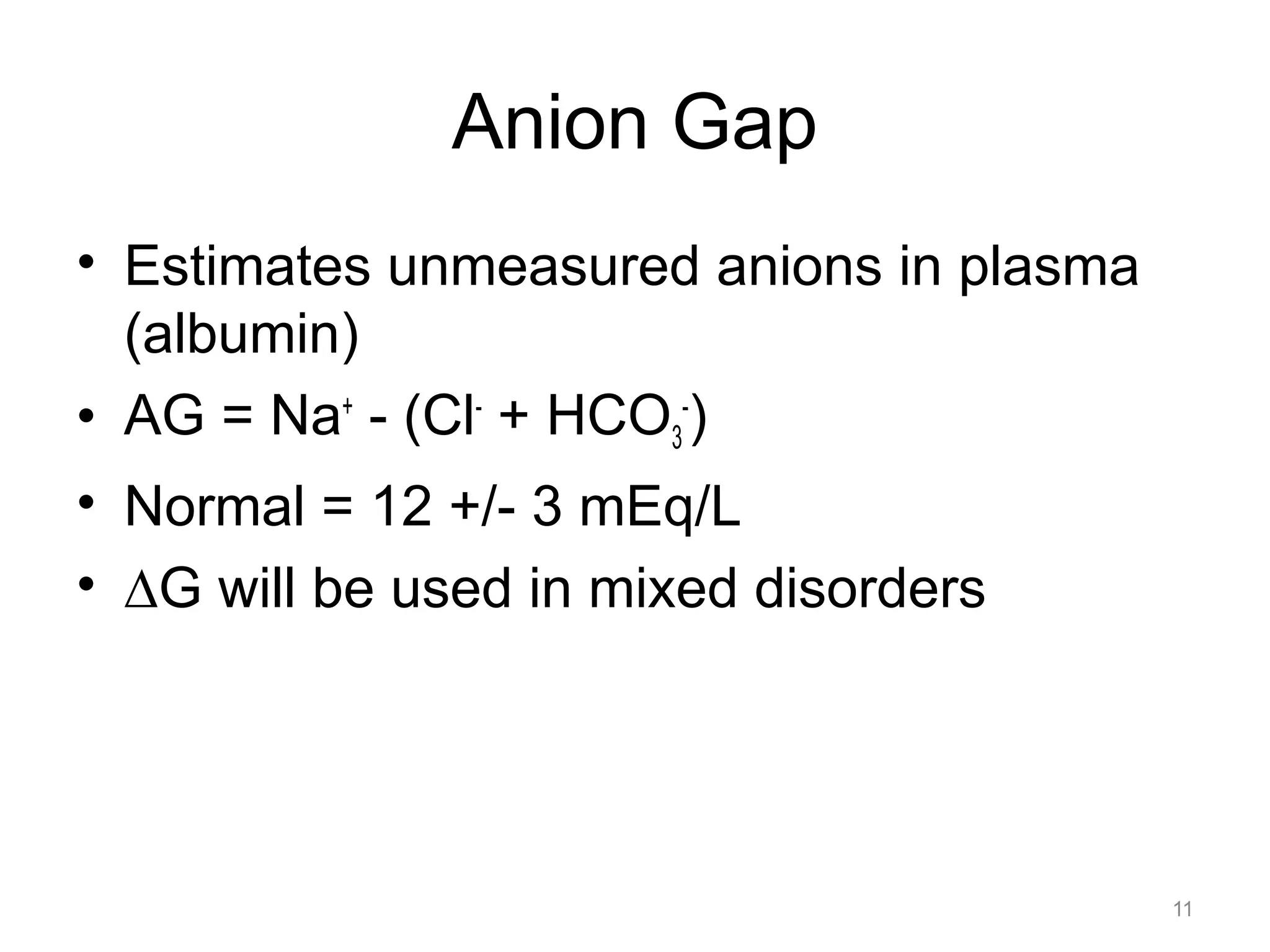

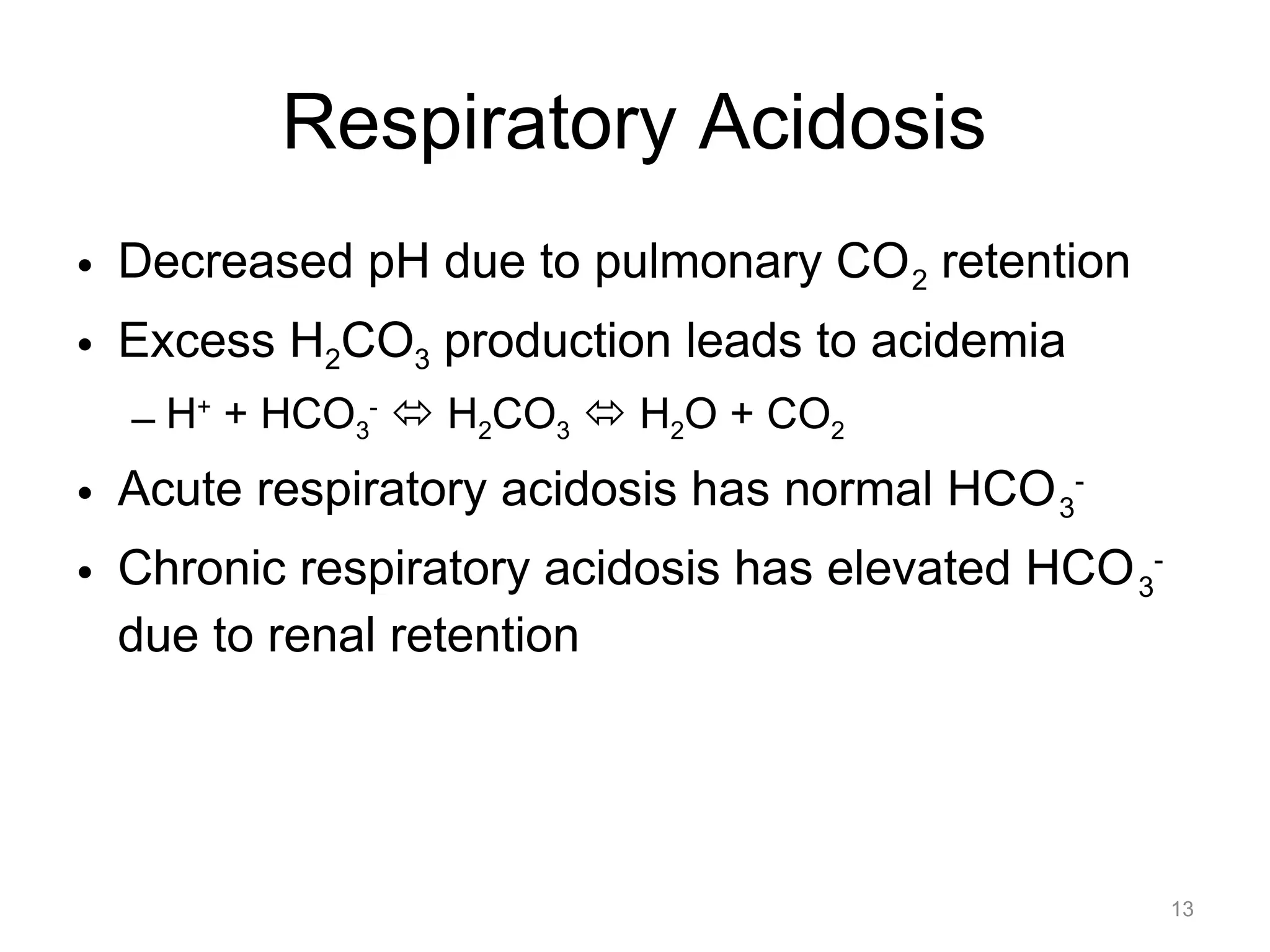



![Respiratory Acidosis
Compensation
• Would you expect the [HCO3-] to increase
or decrease when PaCO2 increases?
17](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-17-2048.jpg)
![Respiratory Acidosis
Compensation
• Would you expect the [HCO3-] to increase
or decrease when PaCO2 increases?
• H+ + HCO3- " H2CO3 " H2O + CO2
18](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-18-2048.jpg)
![Respiratory Acidosis
Compensation
• Acute
– HCO3- production from intracellular proteins
– [HCO3-] increases 1mEq/L for every 10mm Hg rise
in PaCO2
• Chronic
– Renal retention of HCO3– [HCO3-] increases 3.5mEq/L for every 10mm Hg
rise in PaCO2
– Takes 12 hours to many days for renal retention of
HCO3– Nearly normalizes pH
19](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-19-2048.jpg)
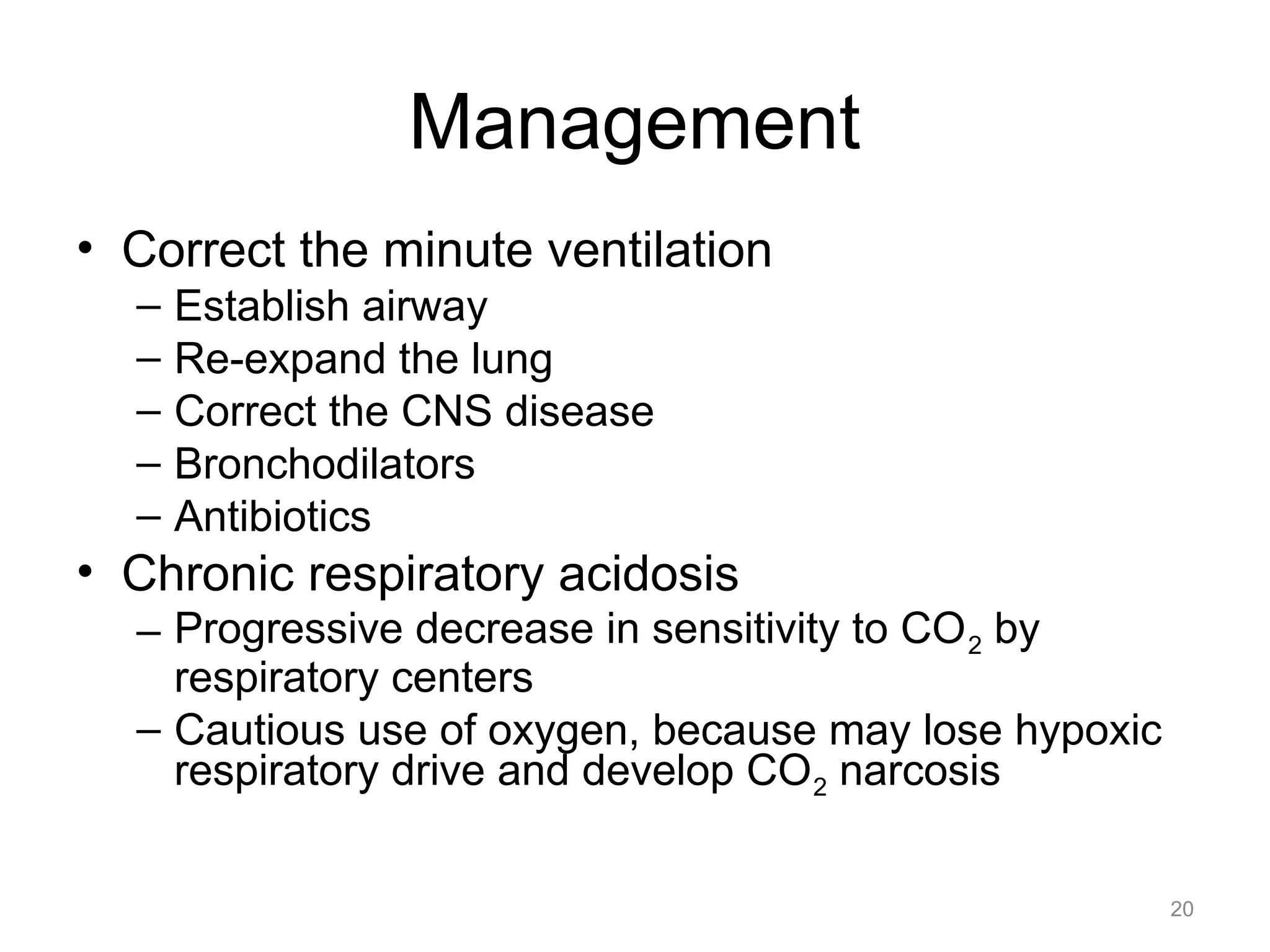

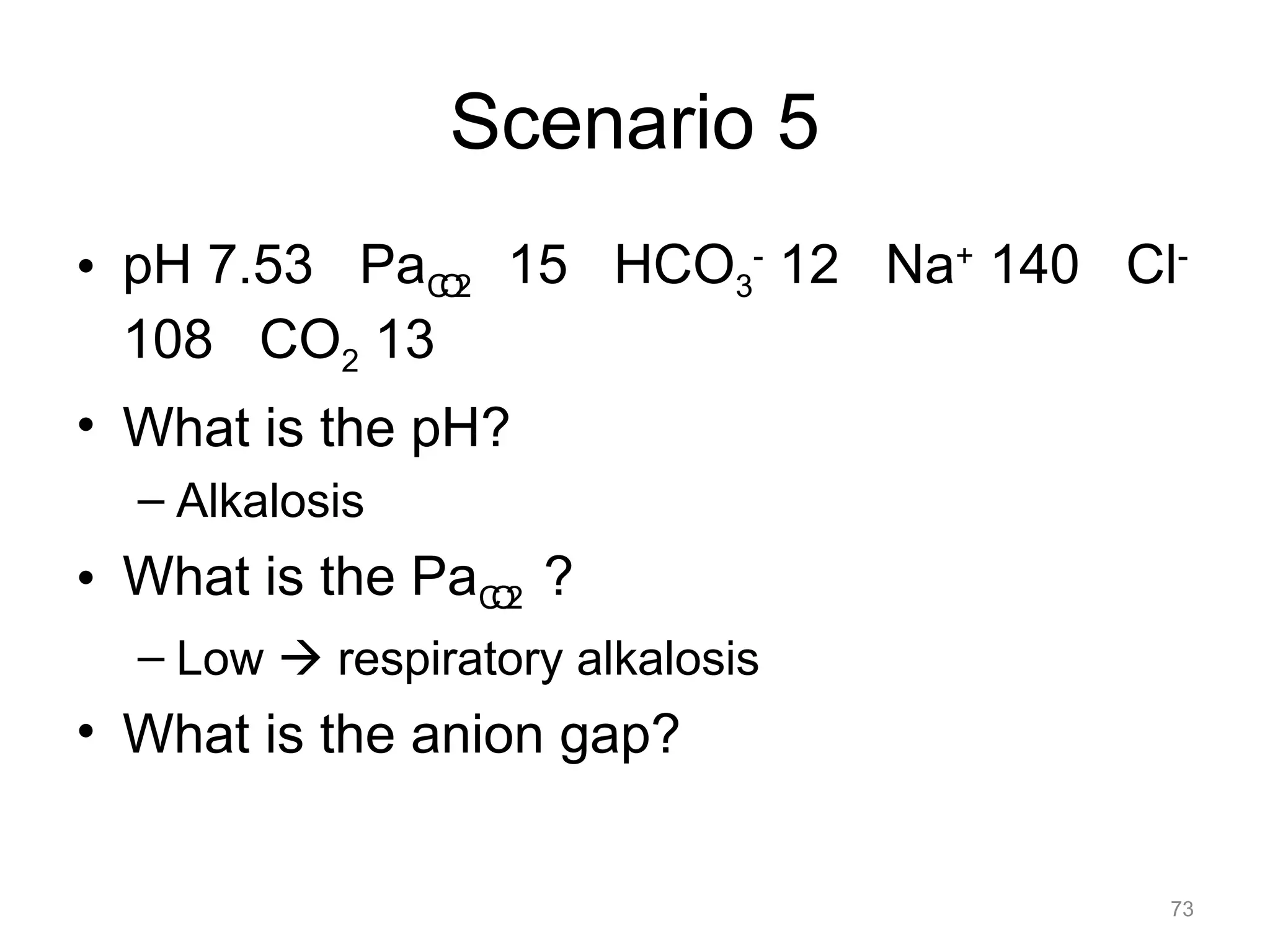
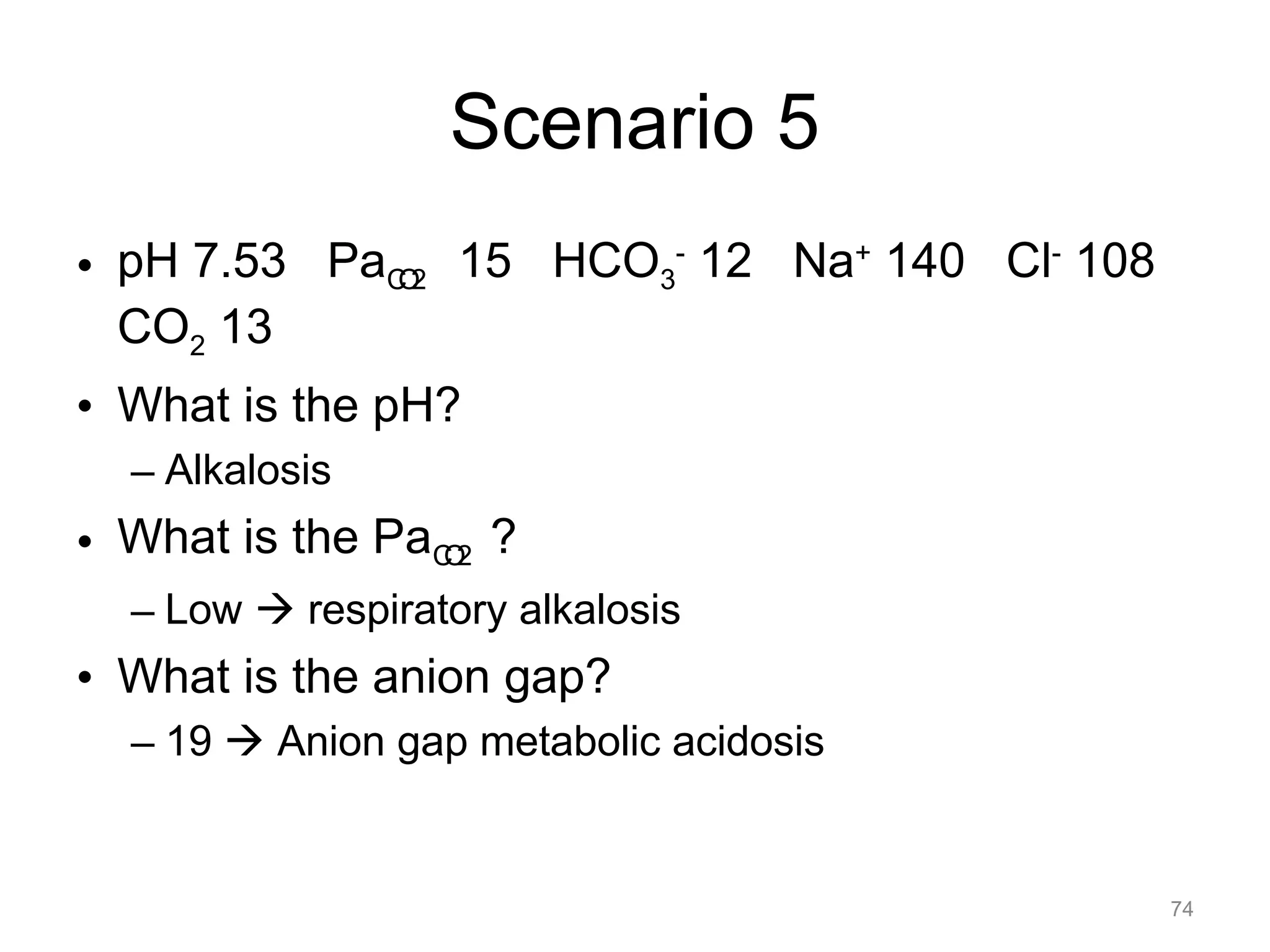
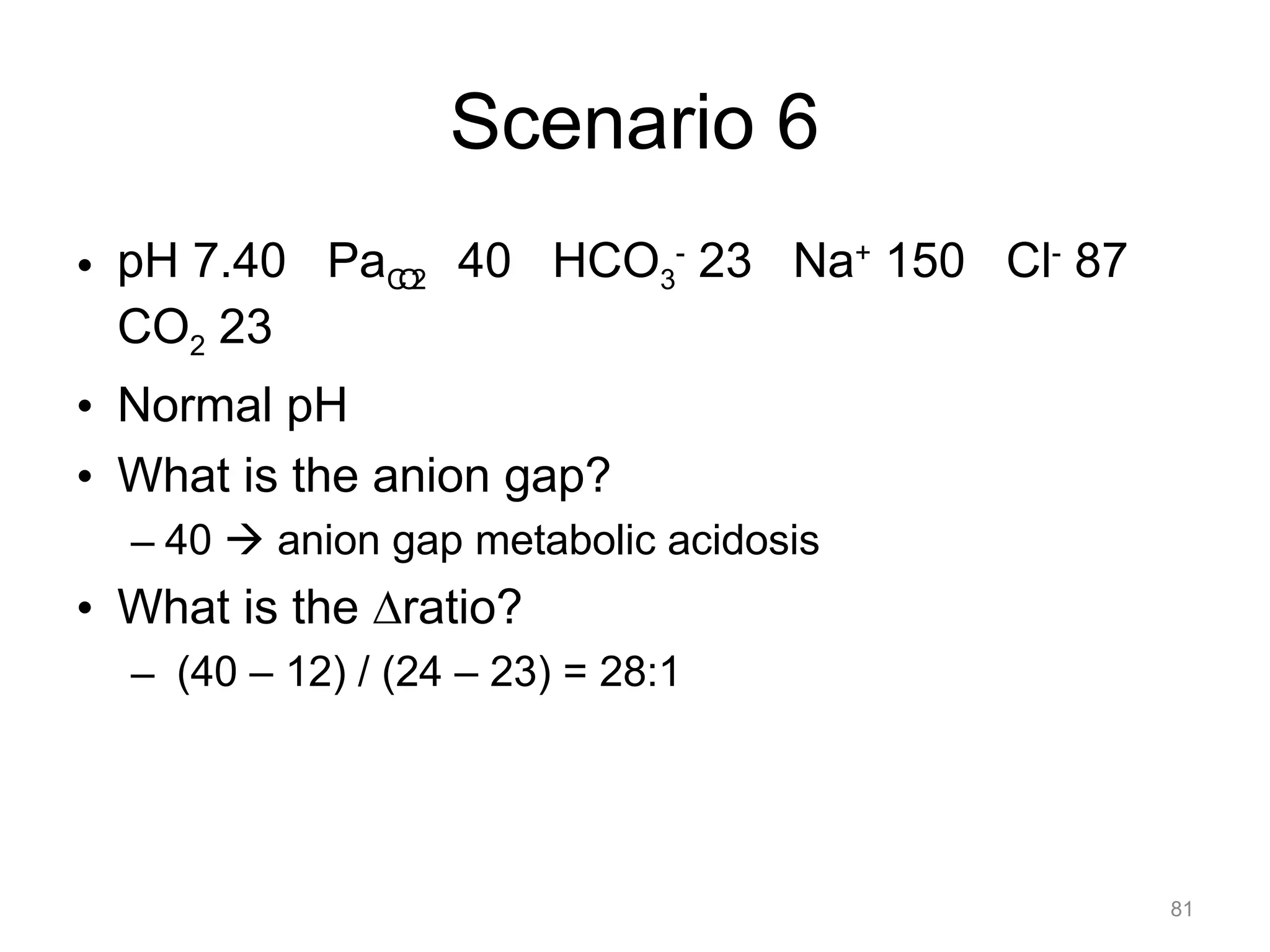
![What is this Acid-Base
Disorder?
• 25 year-old male, heroin overdose
– pH 7.10 PaCO2 80 HCO3- 24
– Acidemic, PaCO2 is elevated, acute change
– Acute respiratory acidosis ([HCO 3-]
unchanged)
22](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-22-2048.jpg)



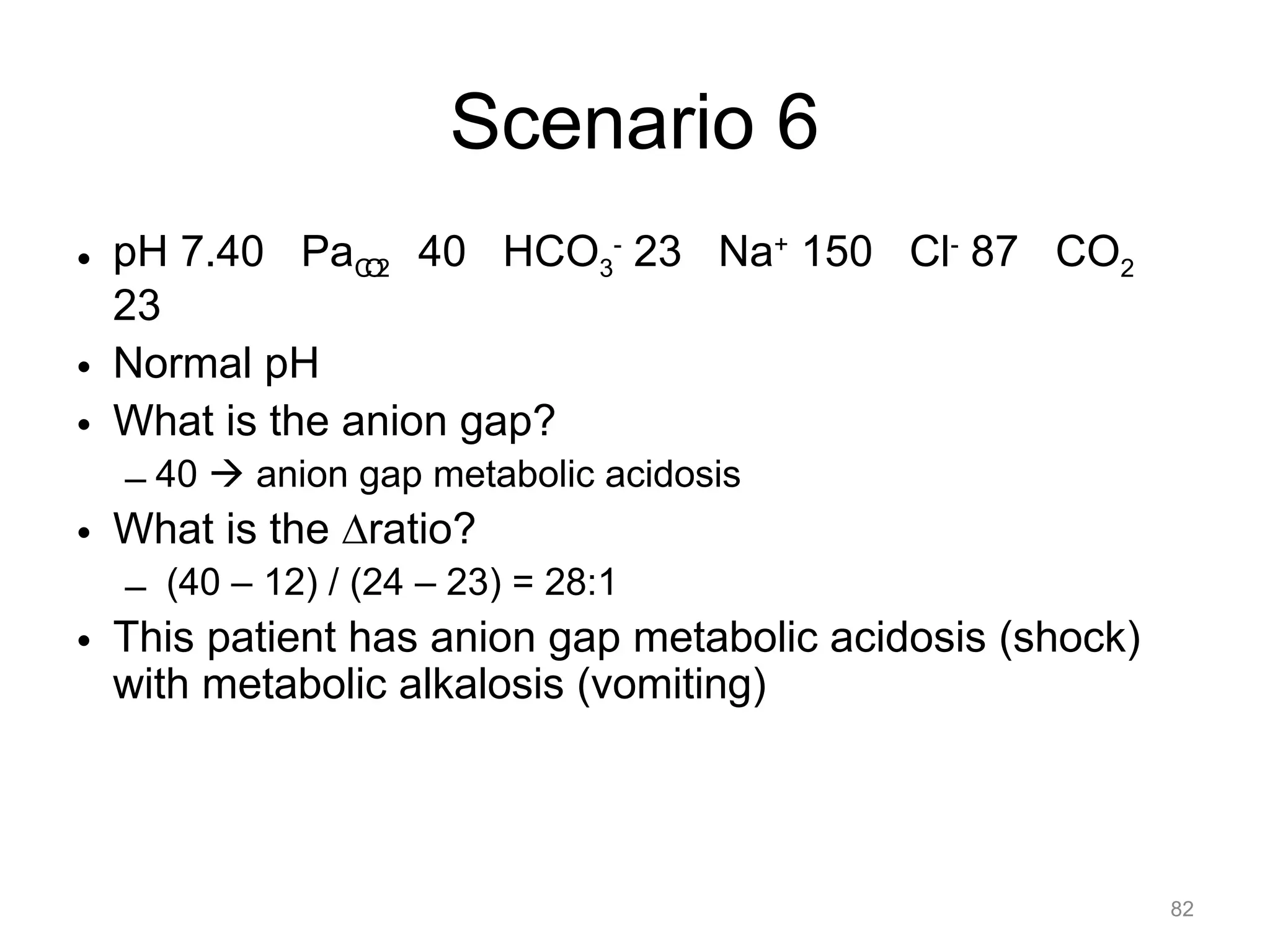
![What is this Acid-Base
Disorder?
• 55 year-old man with COPD
– pH 7.32 PaCO2 70 HCO3- 35
– Acidemic, PaCO2 is elevated ! respiratory
acidosis
– Is the bicarb what you would expect?
– PaCO2 increased by 30, so would expect [HCO 3-]
to increase by 10.5 (3.5 x 3)
26](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-26-2048.jpg)
![What is this Acid-Base
Disorder?
• 55 year-old man with COPD
– pH 7.32 PaCO2 70 HCO3- 35
– Acidemic, PaCO2 is elevated ! respiratory
acidosis
– Is the bicarb what you would expect?
– PaCO2 increased by 30, so would expect [HCO 3-]
to increase by 10.5 (3.5 x 3)
– Yes, it is what you would expect
27](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-27-2048.jpg)



![What is this Acid-Base
Disorder?
• 55 year-old man with COPD
– pH 7.23 PaCO2 90 HCO3- 35
– Acidemic, PaCO2 is elevated ! respiratory
acidosis
– Is the bicarb what you would expect?
– PaCO2 increased by 50, so would expect [HCO 3-]
to increase by 17.5 (3.5 x 5)
31](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-31-2048.jpg)
![What is this Acid-Base
Disorder?
• 55 year-old man with COPD
– pH 7.23 PaCO2 90 HCO3- 35
– Acidemic, Pa CO2 is elevated ! respiratory acidosis
– Is the bicarb what you would expect?
– PaCO2 increased by 50, so would expect [HCO 3-] to increase
by 17.5 (3.5 x 5)
– No, the bicarb has not compensated appropriately yet,
indicating an acute respiratory acidosis on a chronic
respiratory acidosis
32](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-32-2048.jpg)





![Respiratory Alkalosis
Compensation
• Would you expect the [HCO3-] to increase
or decrease when PaCO2 decreases?
38](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-38-2048.jpg)
![Respiratory Alkalosis
Compensation
• Acute
– Plasma [HCO3-] is lowered by 2mEq/L for
every 10-mm Hg decrease in PaCO2
• Chronic
– Plasma [HCO3-] is lowered by 5mEq/L for
every 10-mm Hg decrease in PaCO2
39](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-39-2048.jpg)




![Metabolic Acidosis
• Acidemia created by increase in [H+] or
decrease in [HCO3-]
• Compensated for by hyperventilation to
reduce PaCO2
45](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-45-2048.jpg)
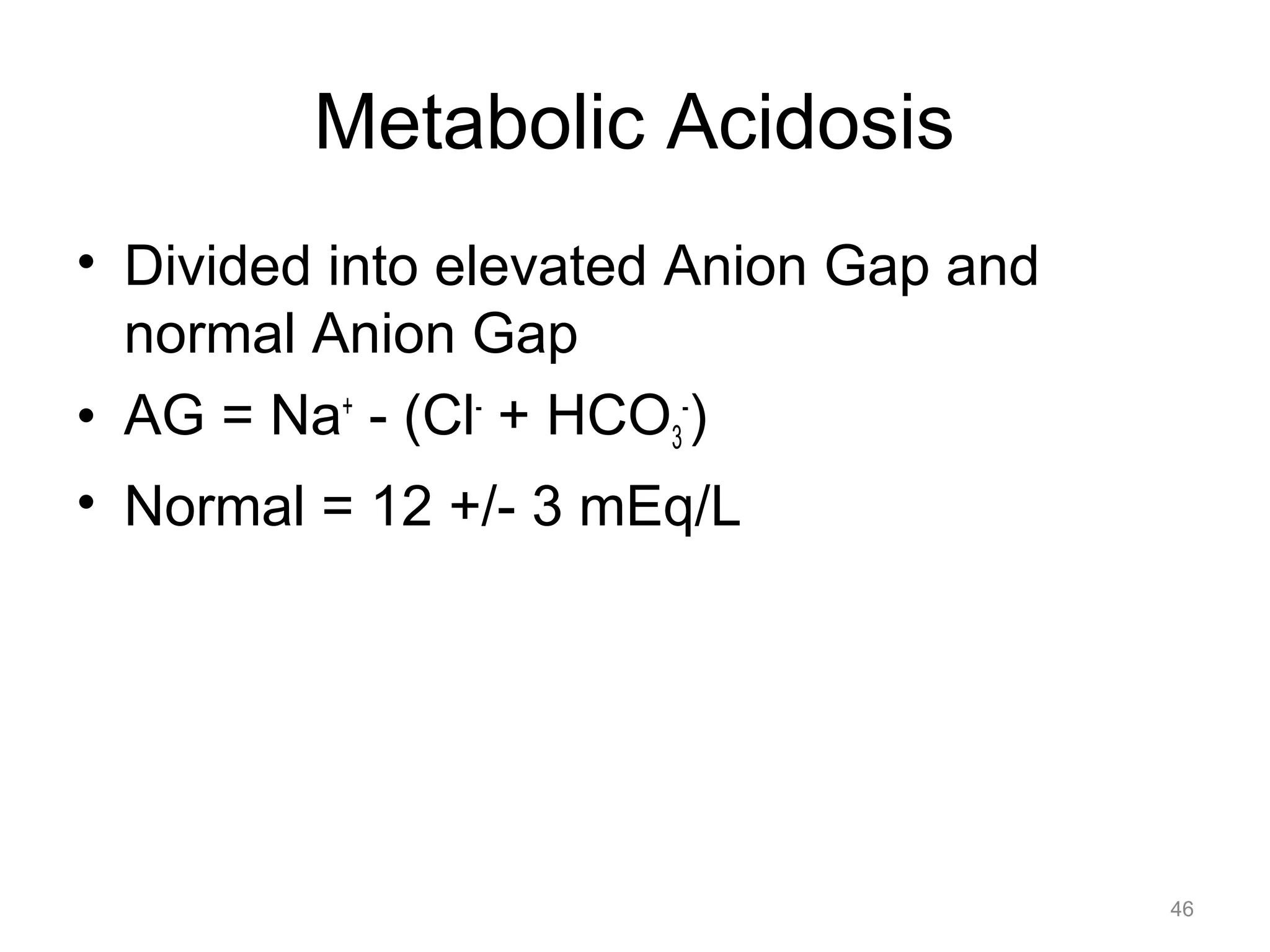


![Metabolic Acidosis
• Compensation (Winter’s Formula)
– PaCO2 = 1.5 x [HCO3-] + 8 +/- 2
= 1.5 x [HCO3-] + 6 or 10
49](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-49-2048.jpg)








![Metabolic Alkalosis
• Alkalemia created by decrease in [H+] or
increase in [HCO3-]
• Compensated for by hypoventilation to
increase PaCO2
58](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-58-2048.jpg)


![Metabolic Alkalosis
• Compensation
– PaCO2 = 0.9 x [HCO3- ] + 15
61](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-61-2048.jpg)





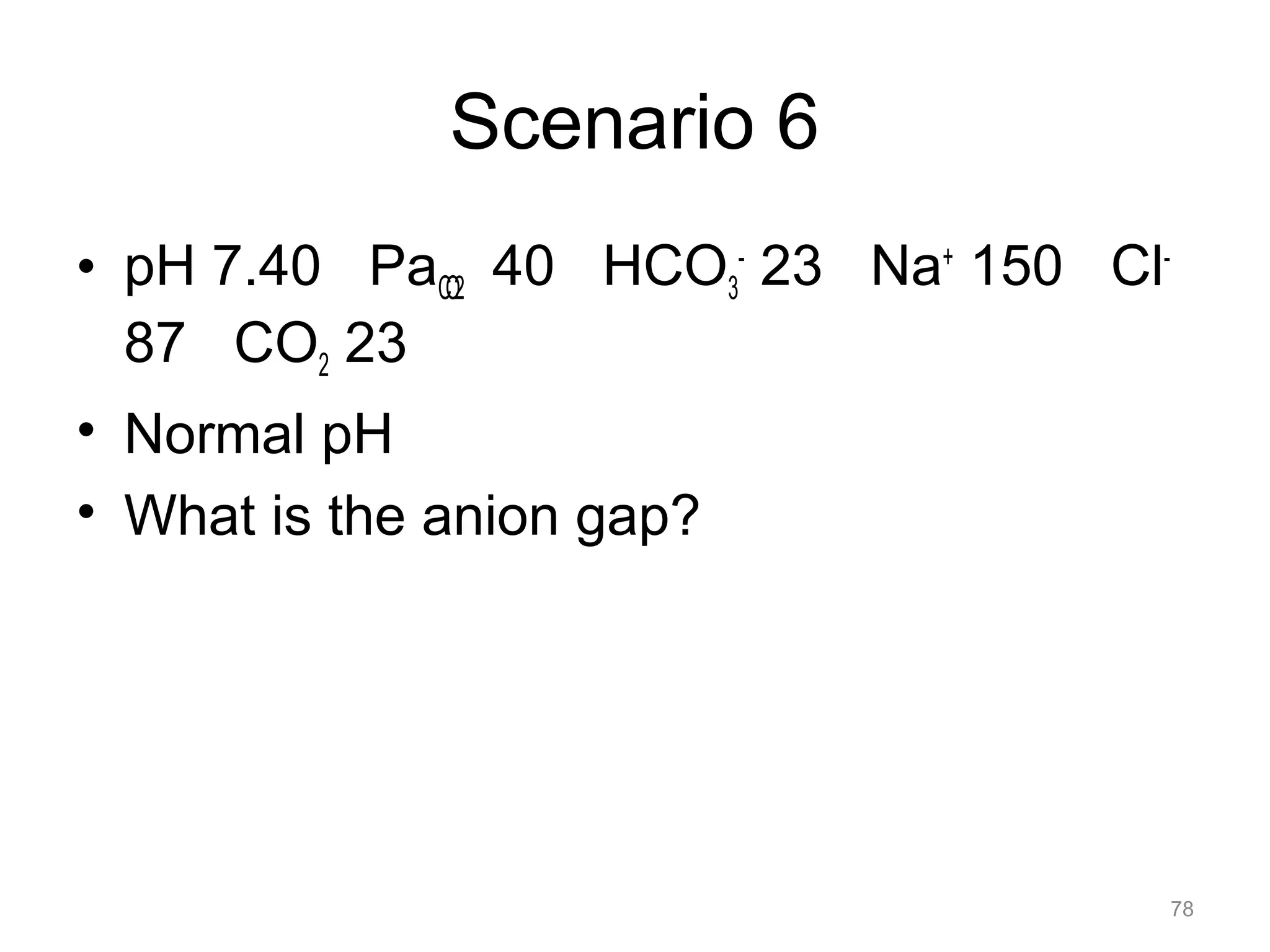
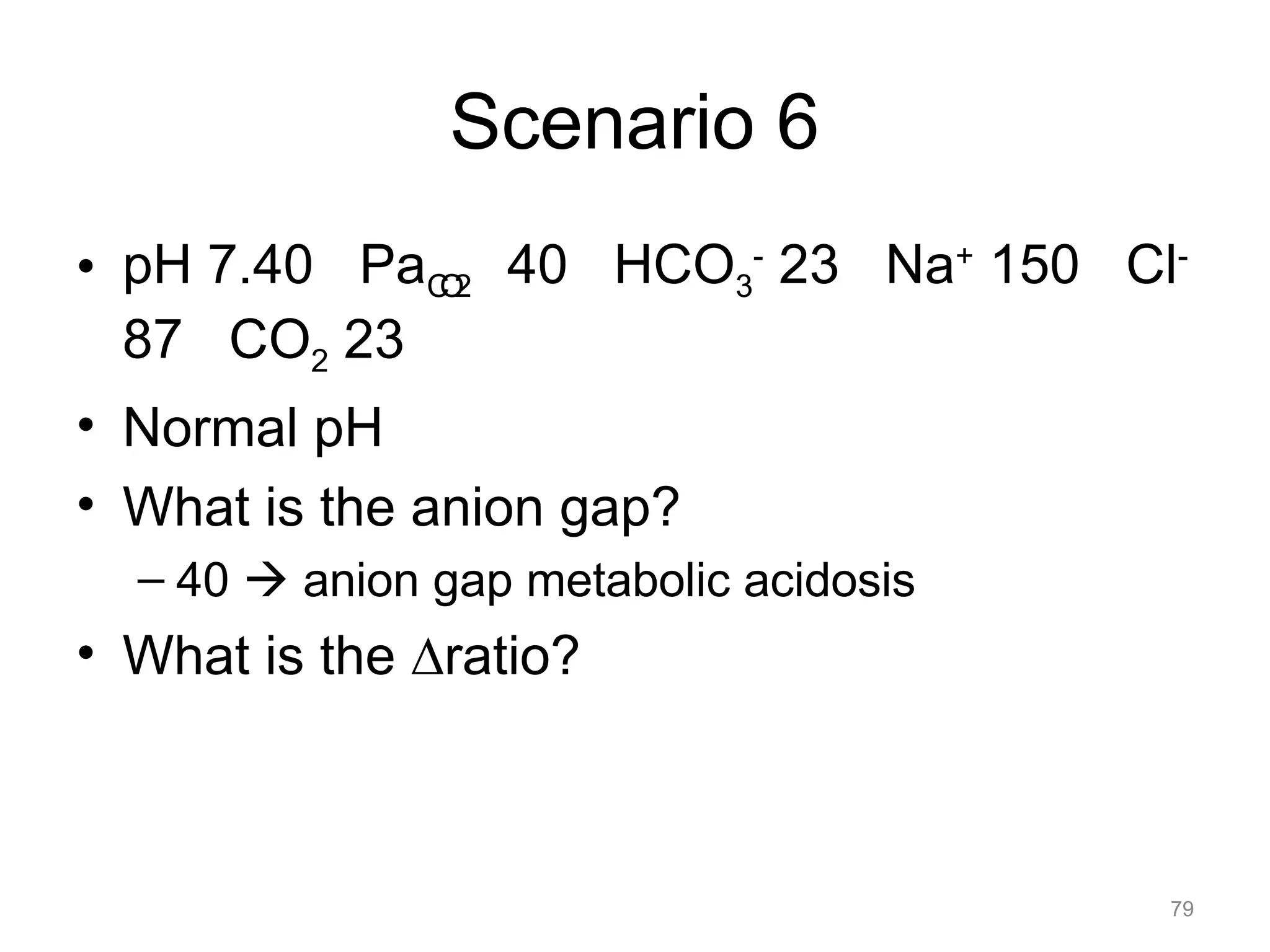
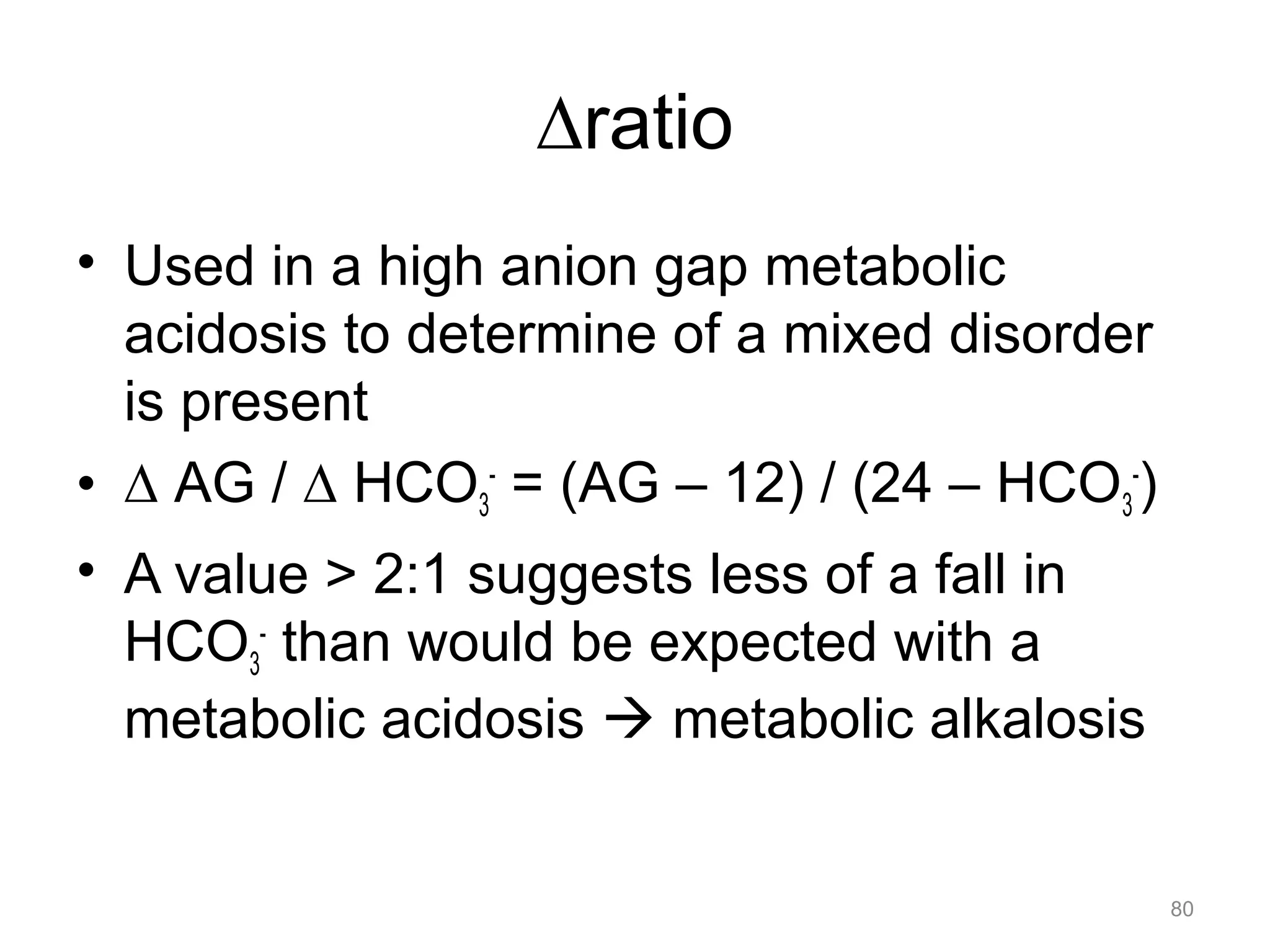
![Mixed Disorders
• If the pH is near normal, and the PaCO2
and/or the [HCO3-] is abnormal, assume a
mixed disorder
67](https://image.slidesharecdn.com/2012-gemc-res-brouwer-acid-basedisorders-oer-140208192104-phpapp02/75/GEMC-Acid-Base-Disorders-for-Residents-67-2048.jpg)