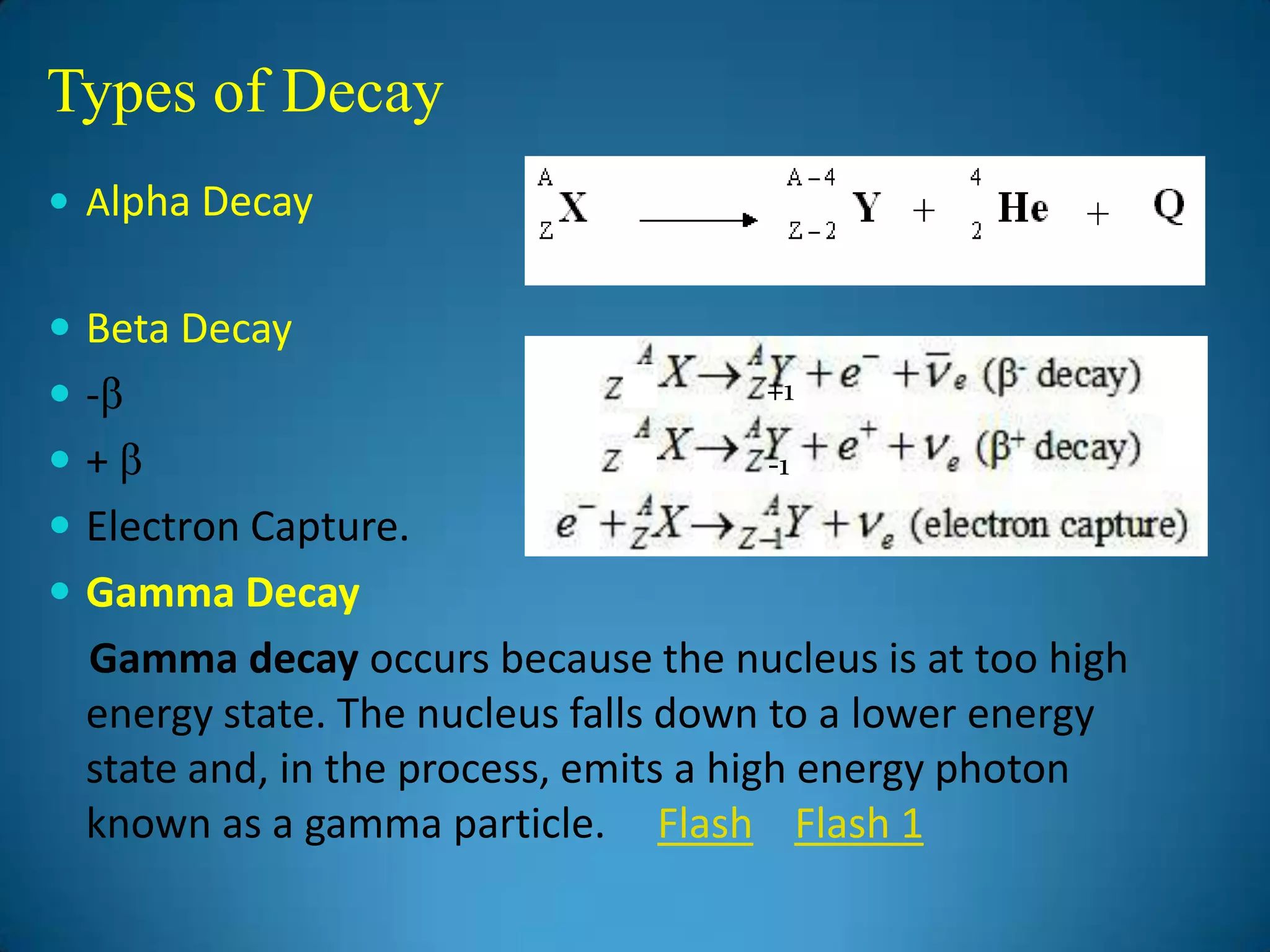
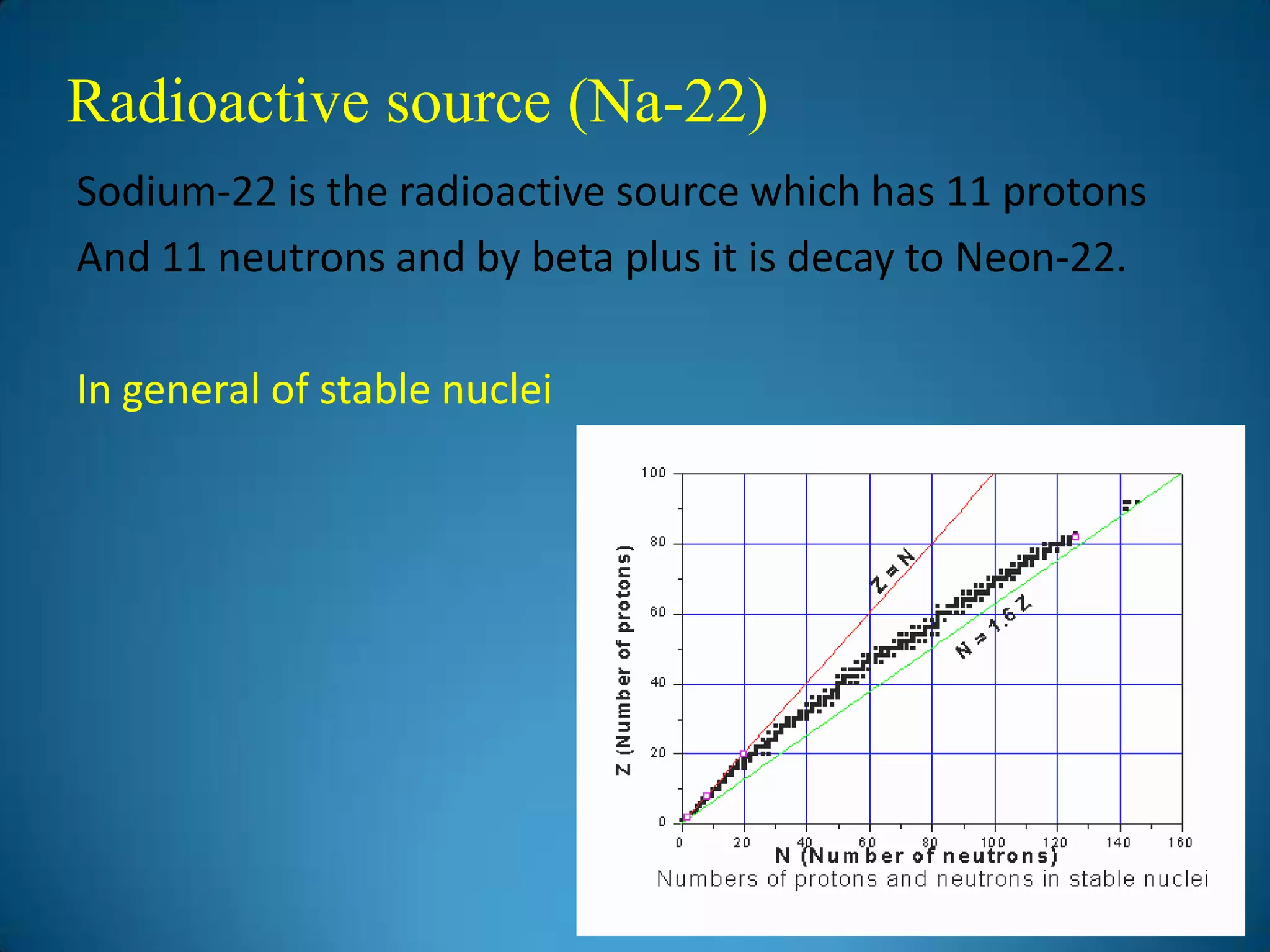
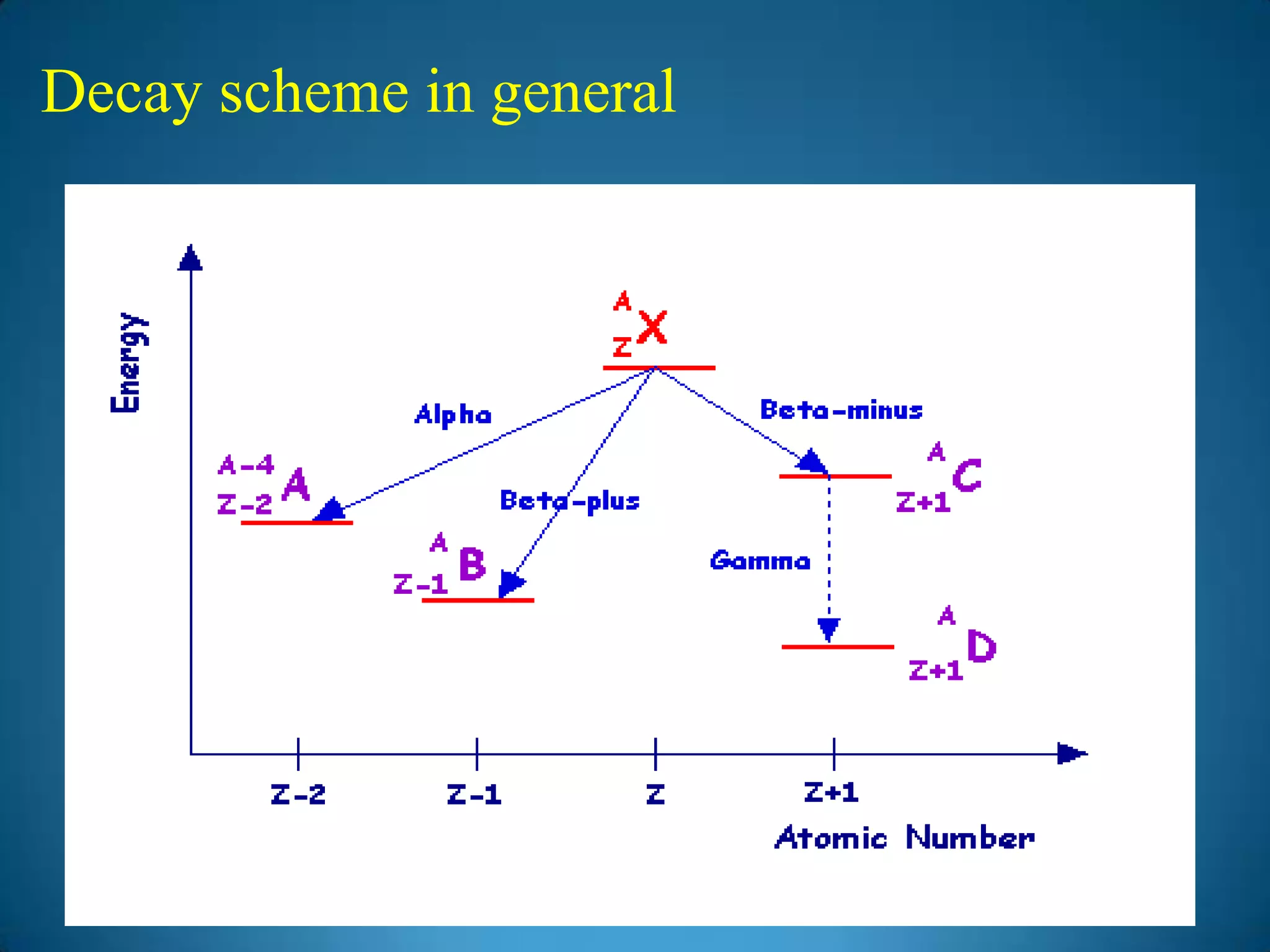
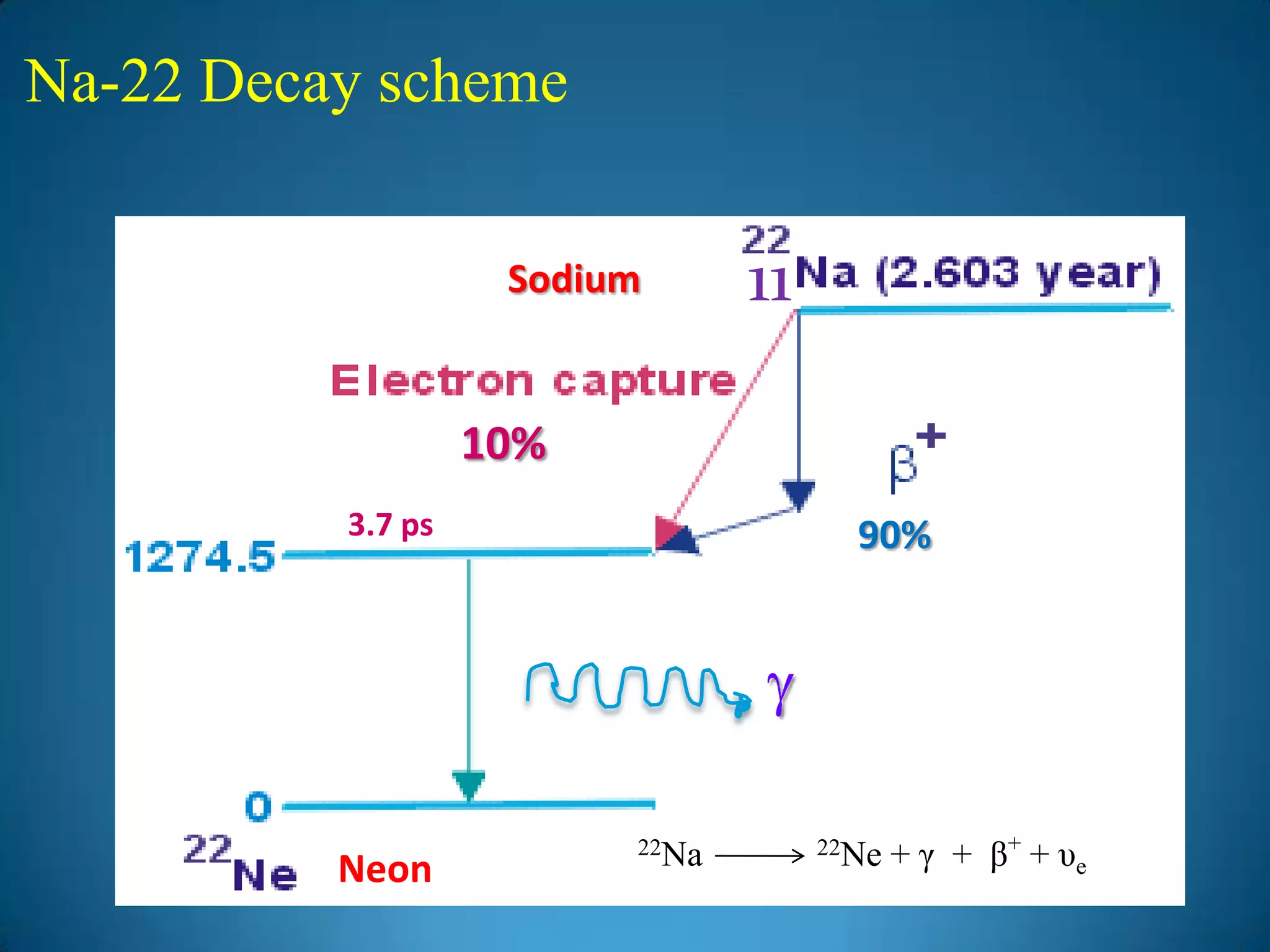
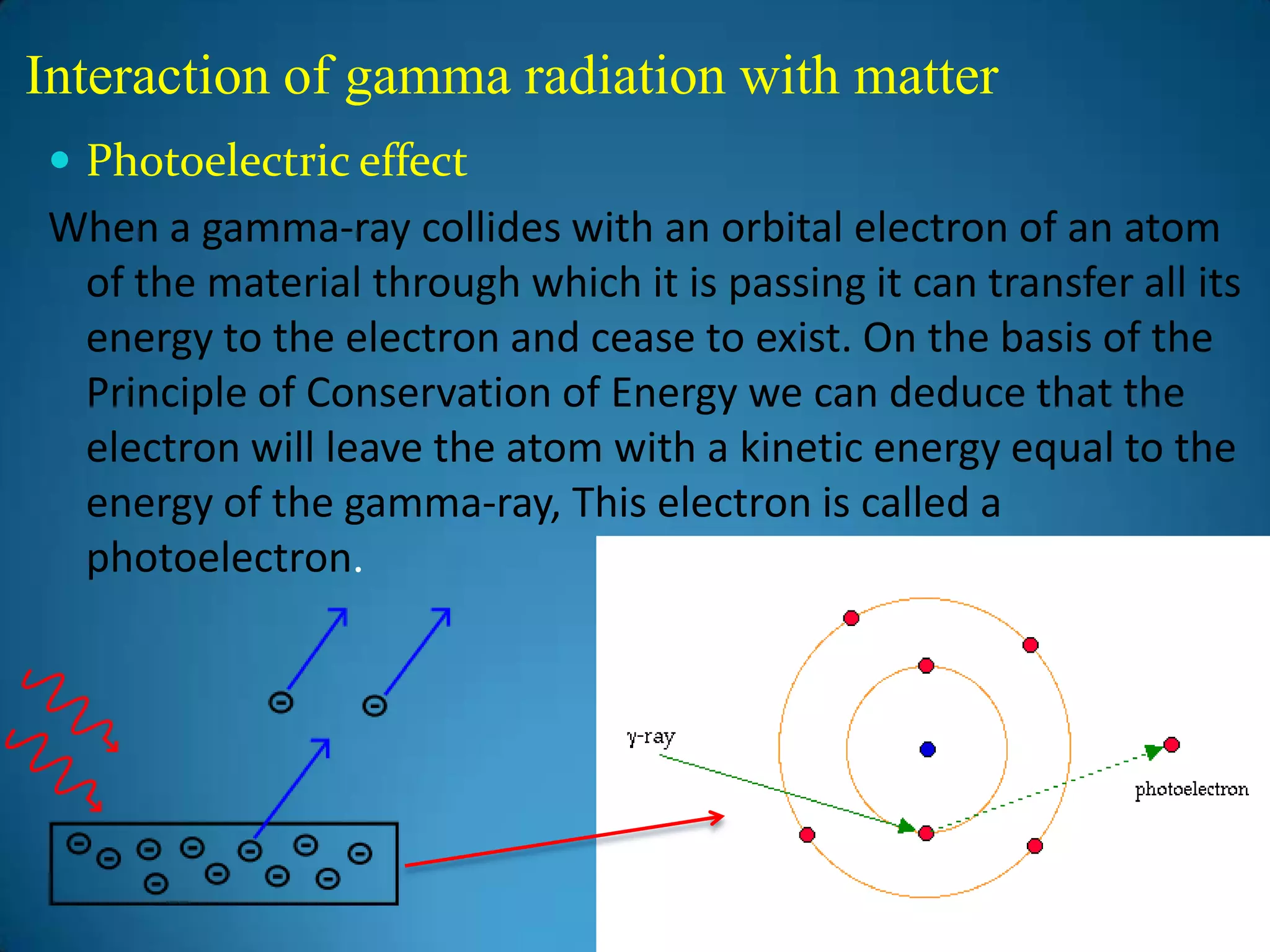
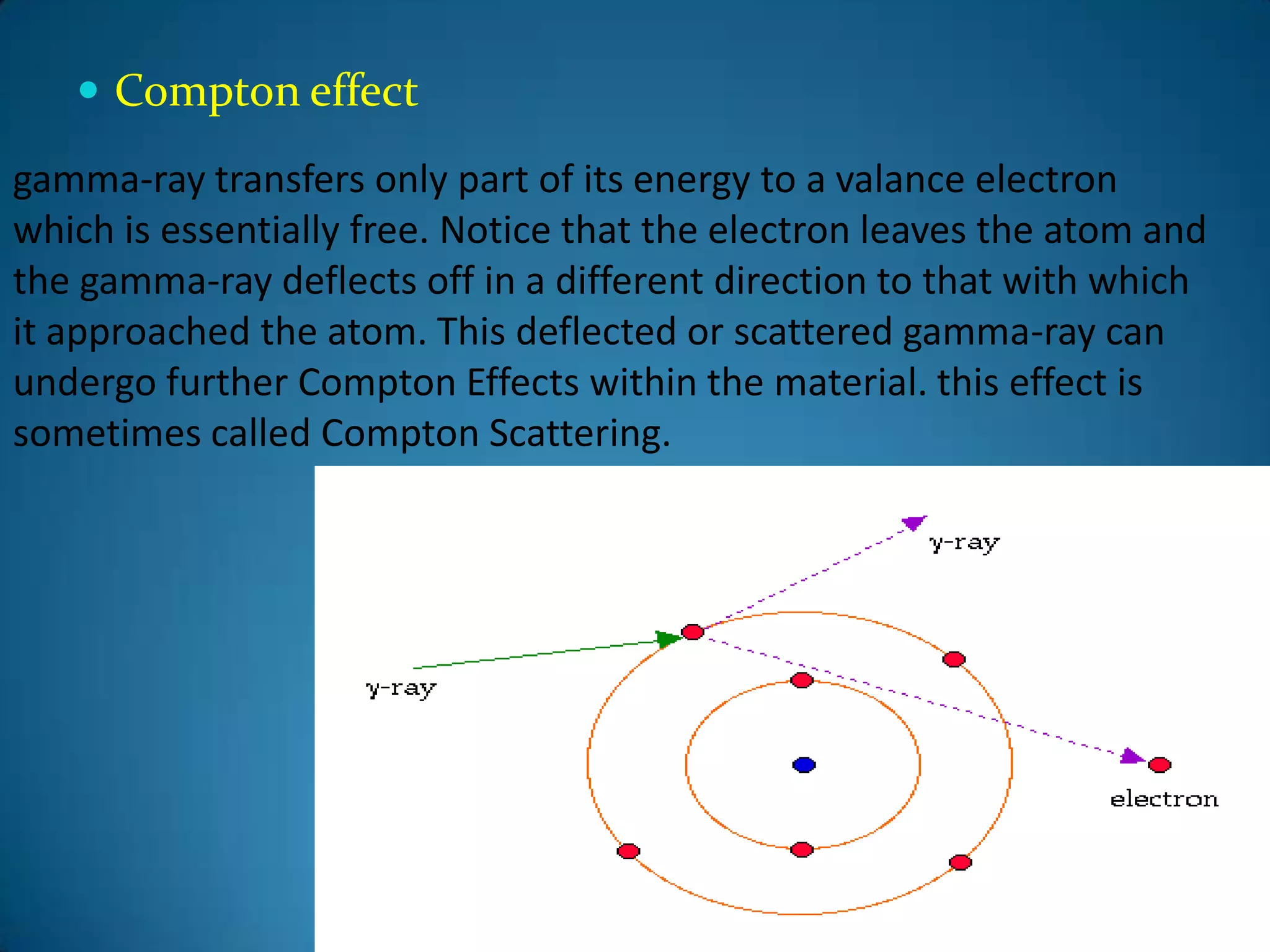
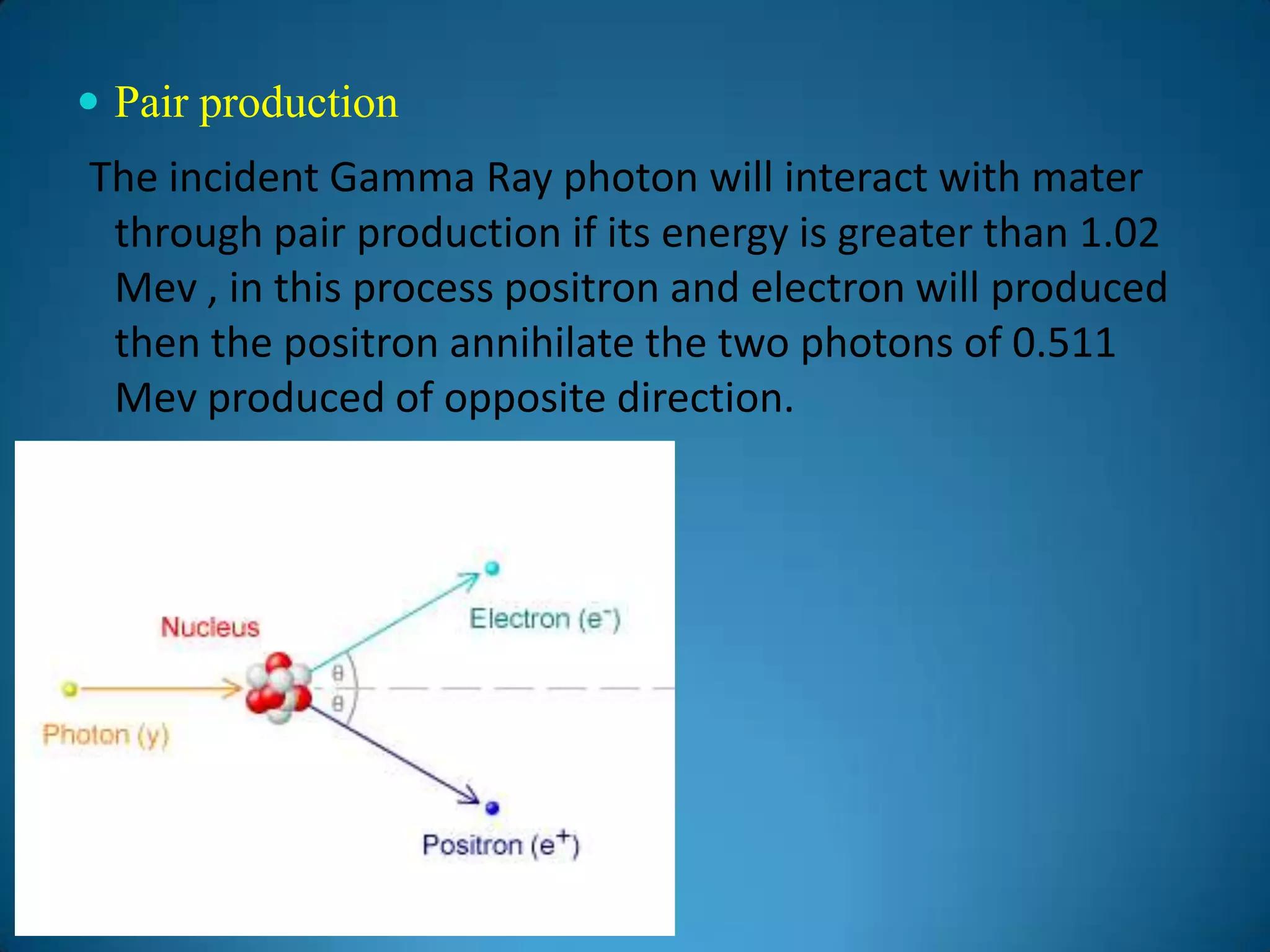
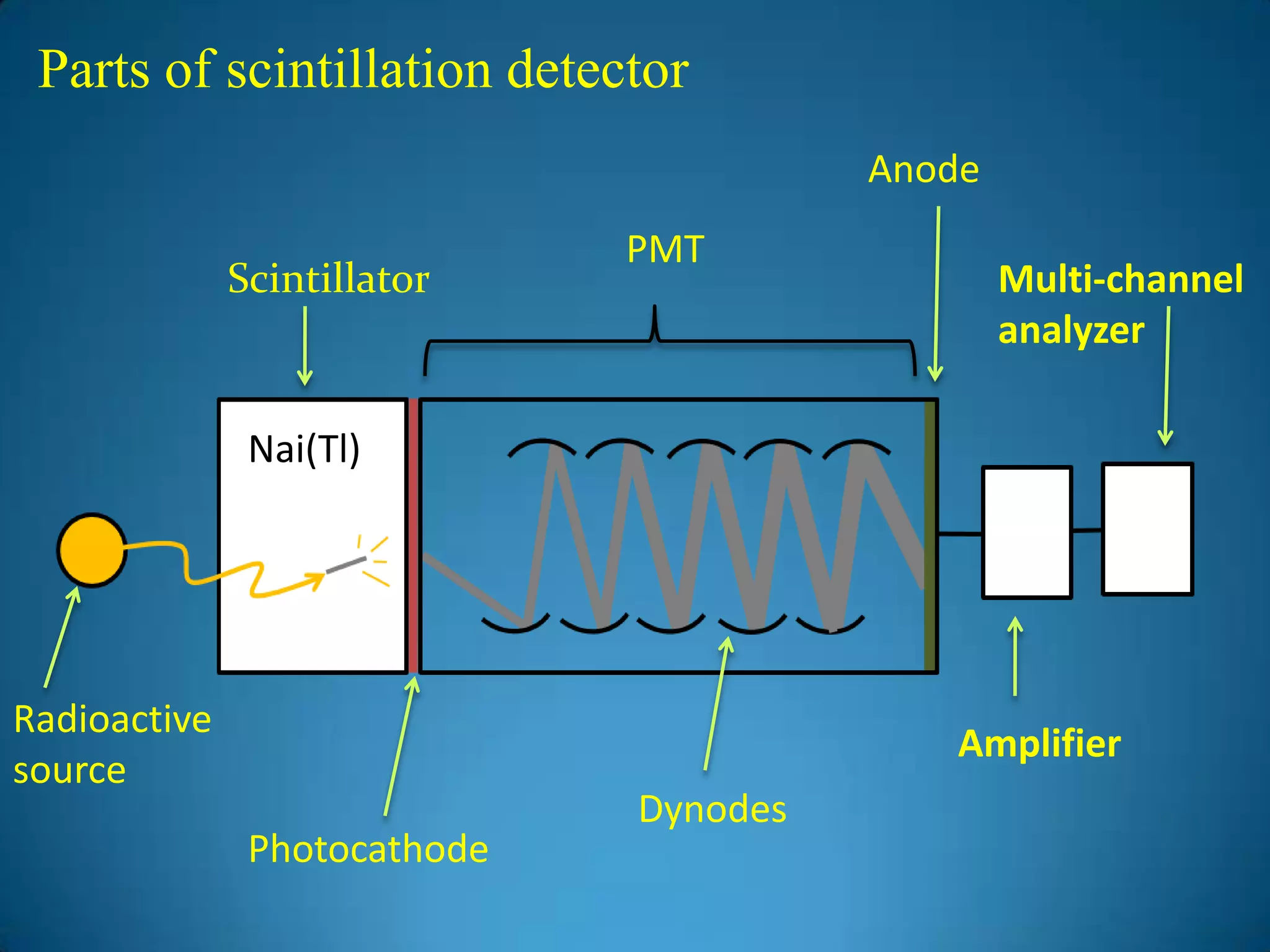
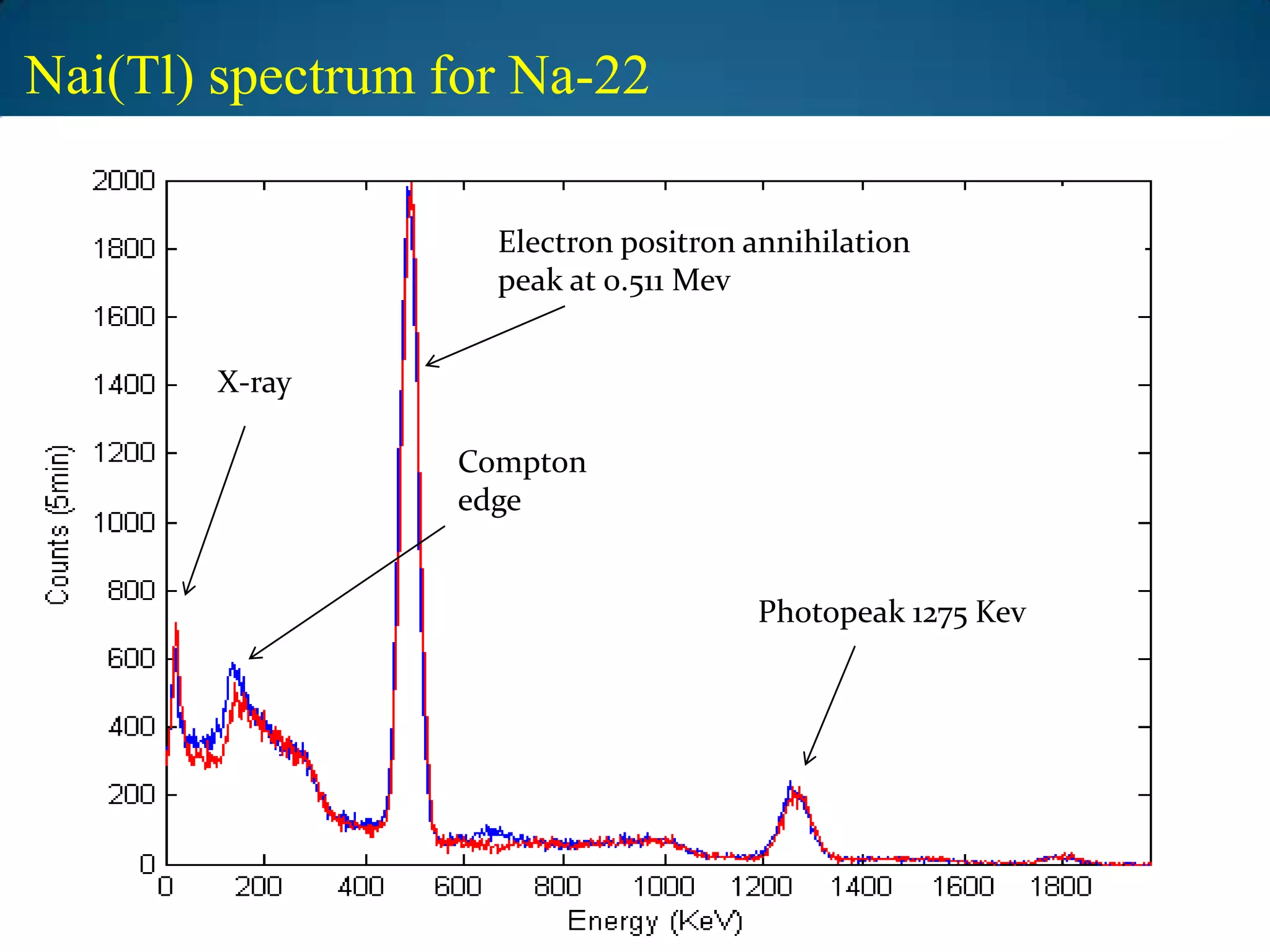
The document discusses the study of gamma-ray spectrum using scintillation detectors and single channel analyzers, highlighting the nature of radioactive decay and transmutation. It explains different types of decay, including gamma decay, and delves into interactions of gamma radiation with matter, such as the photoelectric effect and Compton scattering. Additionally, it describes the properties and functioning of scintillation detectors, particularly thallium-activated sodium iodide (NaI(Tl)).