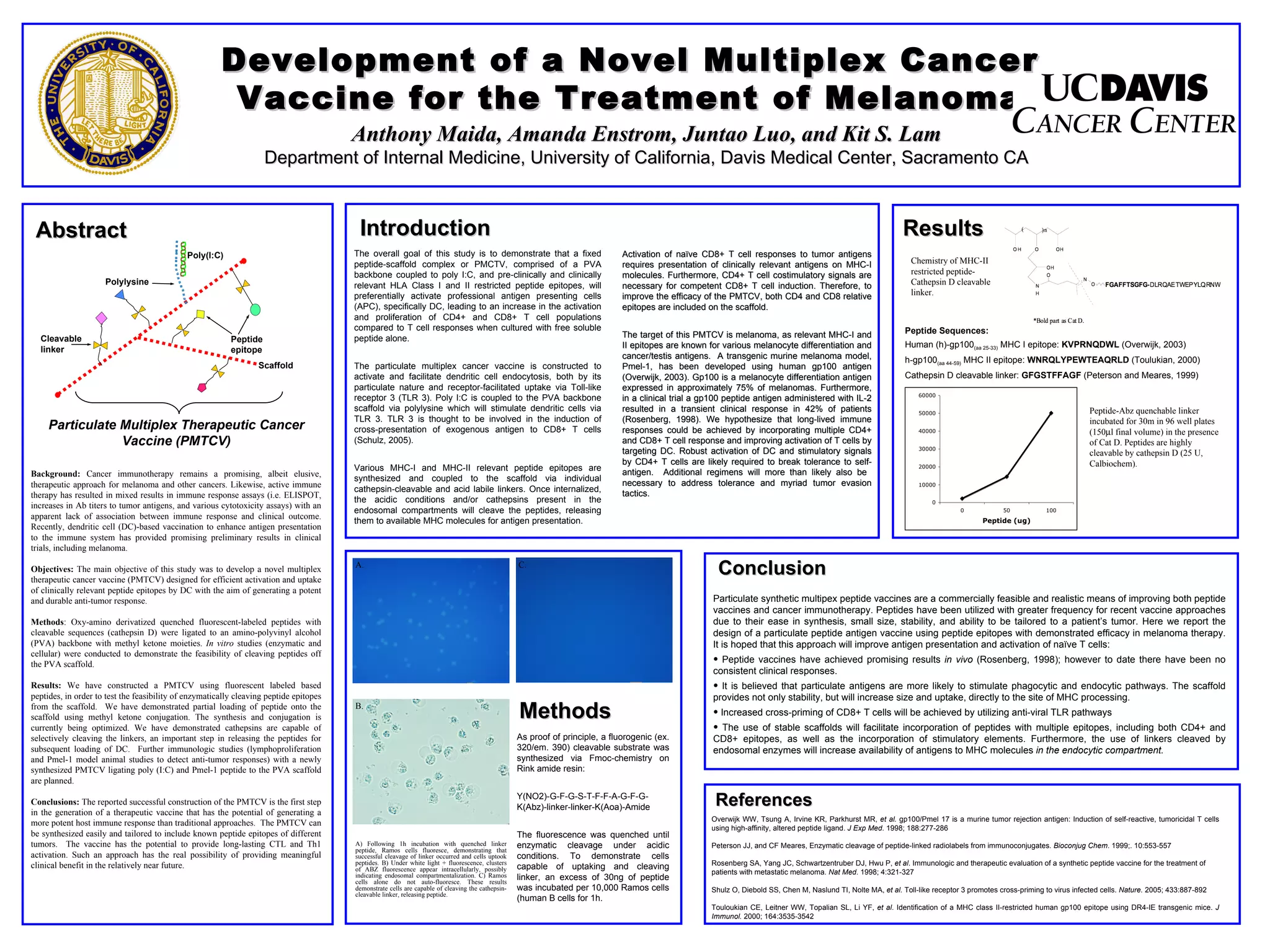
1) The document describes the development of a novel multiplex cancer vaccine for melanoma composed of a polyvinyl alcohol (PVA) backbone coupled to poly(I:C) and clinically relevant HLA Class I and II restricted peptide epitopes from melanoma antigens like gp100.
2) In vitro experiments demonstrated that cells could uptake and cleave fluorescent-labeled peptides from the scaffold via acid-labile linkers, releasing the peptides.
3) The particulate vaccine aims to preferentially activate dendritic cells compared to free peptides alone, in order to generate a potent anti-tumor T cell response for melanoma immunotherapy.