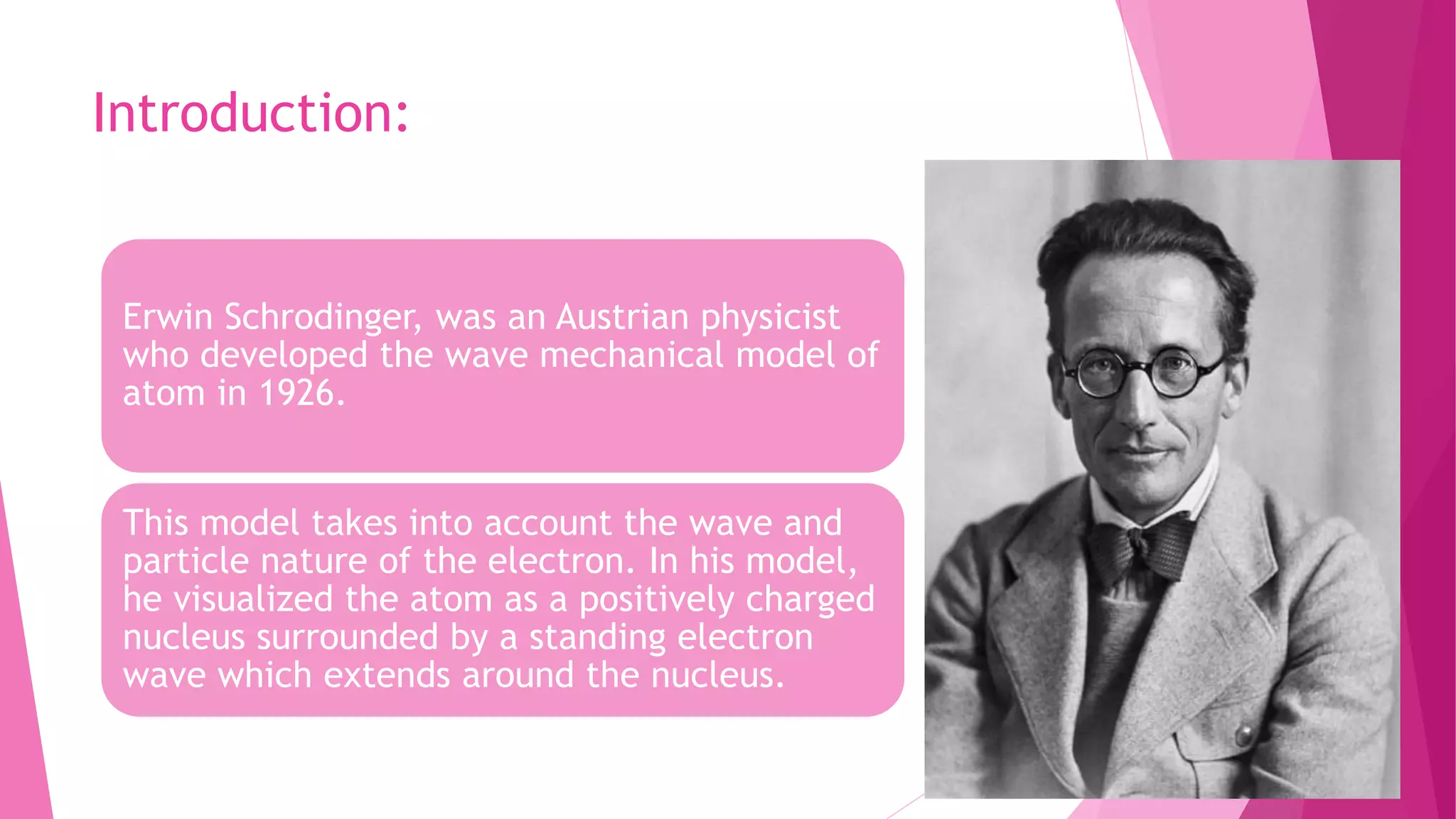
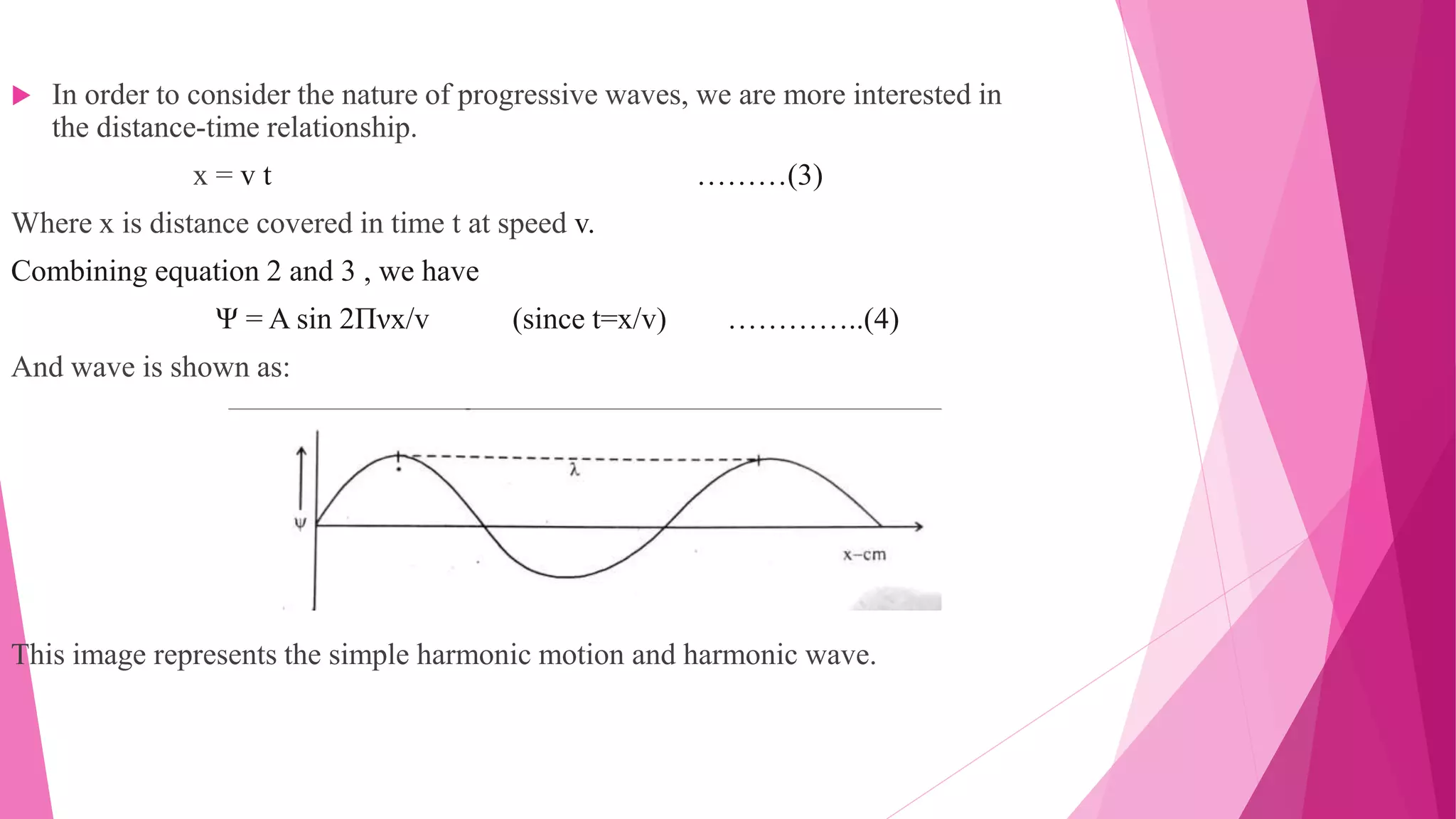
The Schrodinger wave equation is fundamental in quantum mechanics, analogous to Newton's laws in classical mechanics, predicting the behavior of dynamic systems through wave functions. Erwin Schrodinger's model visualizes atoms with electron waves around a nucleus, and the equation is utilized for calculating energy states and wave functions of particles in various systems. Key applications include determining energy levels in atoms and molecules, introducing quantum numbers, and analyzing wave-particle duality in electrons.