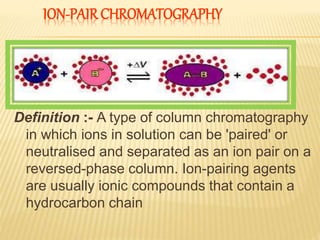
1) Ion pair chromatography is a type of column chromatography that uses ion pairing agents to neutralize charged analytes and allow their separation on a reversed-phase column.
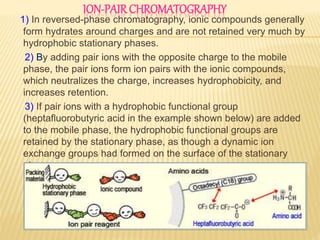
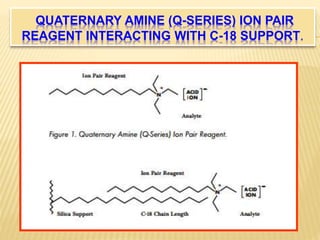
2) By adding counter ions with the opposite charge to the mobile phase, ion pairs form between the counter ions and analytes, neutralizing their charge and increasing their hydrophobicity.
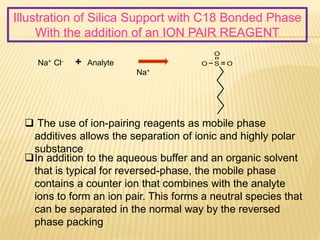
3) The use of ion-pairing reagents as mobile phase additives allows the separation of ionic and highly polar substances that cannot otherwise be separated by reversed-phase chromatography.