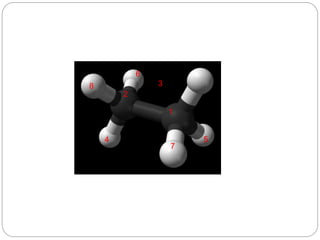
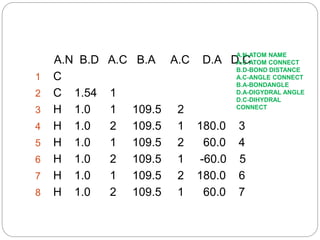
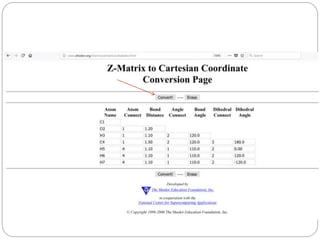
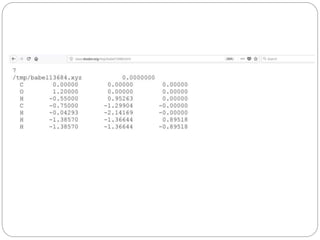
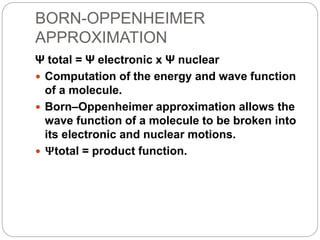
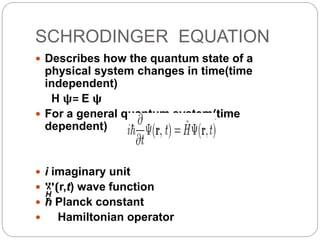
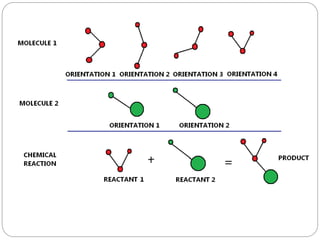
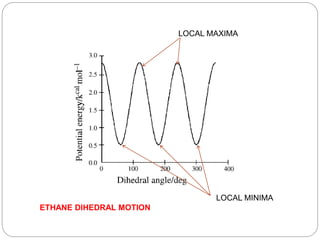
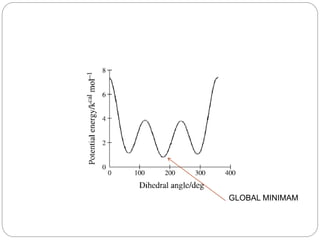
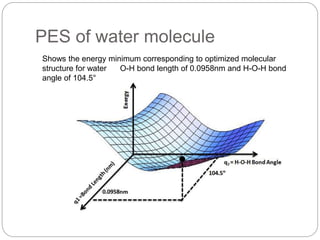
The document discusses Z-matrices and potential energy surfaces. It defines a Z-matrix as a way to represent molecules using internal coordinates like bond lengths, angles, and dihedrals. It then gives an example Z-matrix for methane. It also defines potential energy surfaces as describing the energy of a system, like a collection of atoms, in terms of parameters like atomic positions. It discusses how potential energy surfaces are used to theoretically explore molecular properties and chemical reactions.