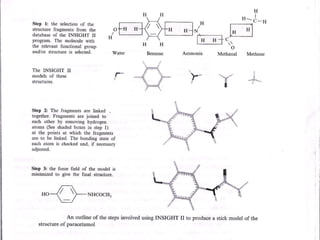
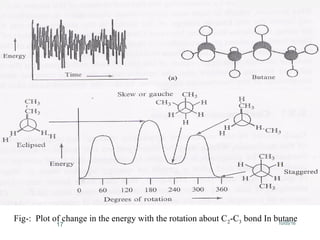
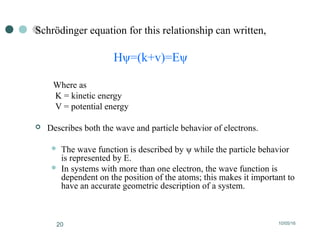
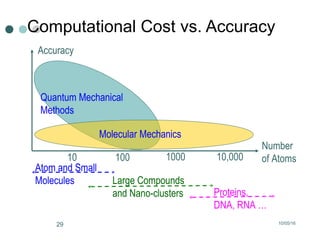
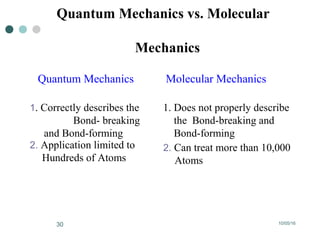
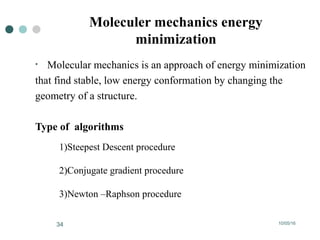
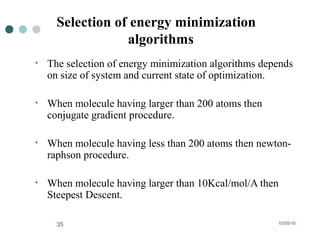
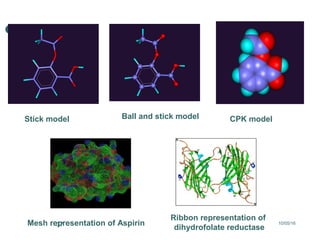
Computational chemistry uses theoretical chemistry calculations incorporated into computer programs to calculate molecular structures and properties. It can calculate properties such as structure, energy, charge distribution, and spectroscopic quantities using methods that range from highly accurate ab initio methods to less accurate semi-empirical and molecular mechanics methods. Computational chemistry allows medicinal chemists to use computer power to measure molecular geometry, electron density, energies, and more for applications such as conformational analysis, docking ligands in receptor sites, and comparing ligands.