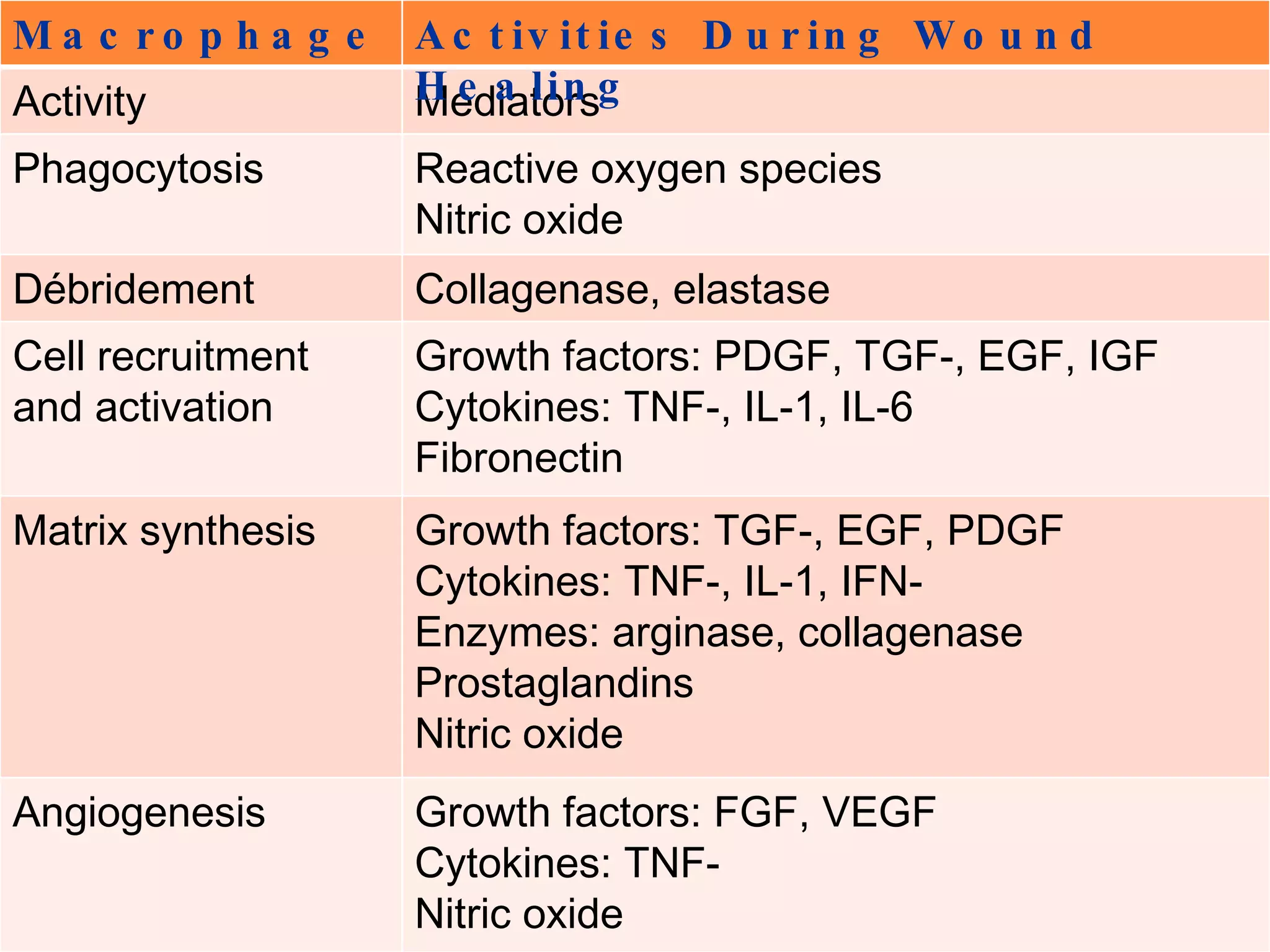
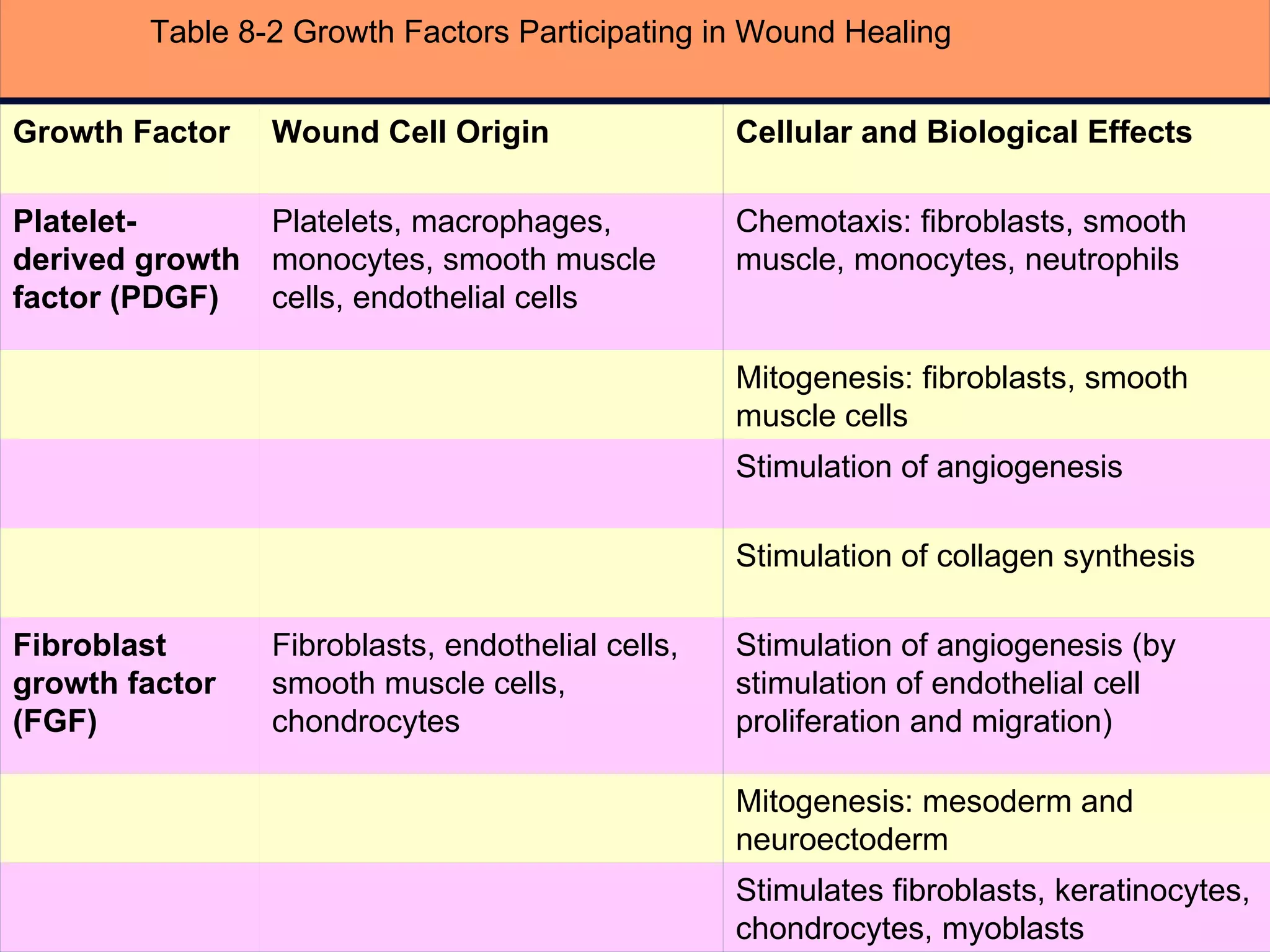
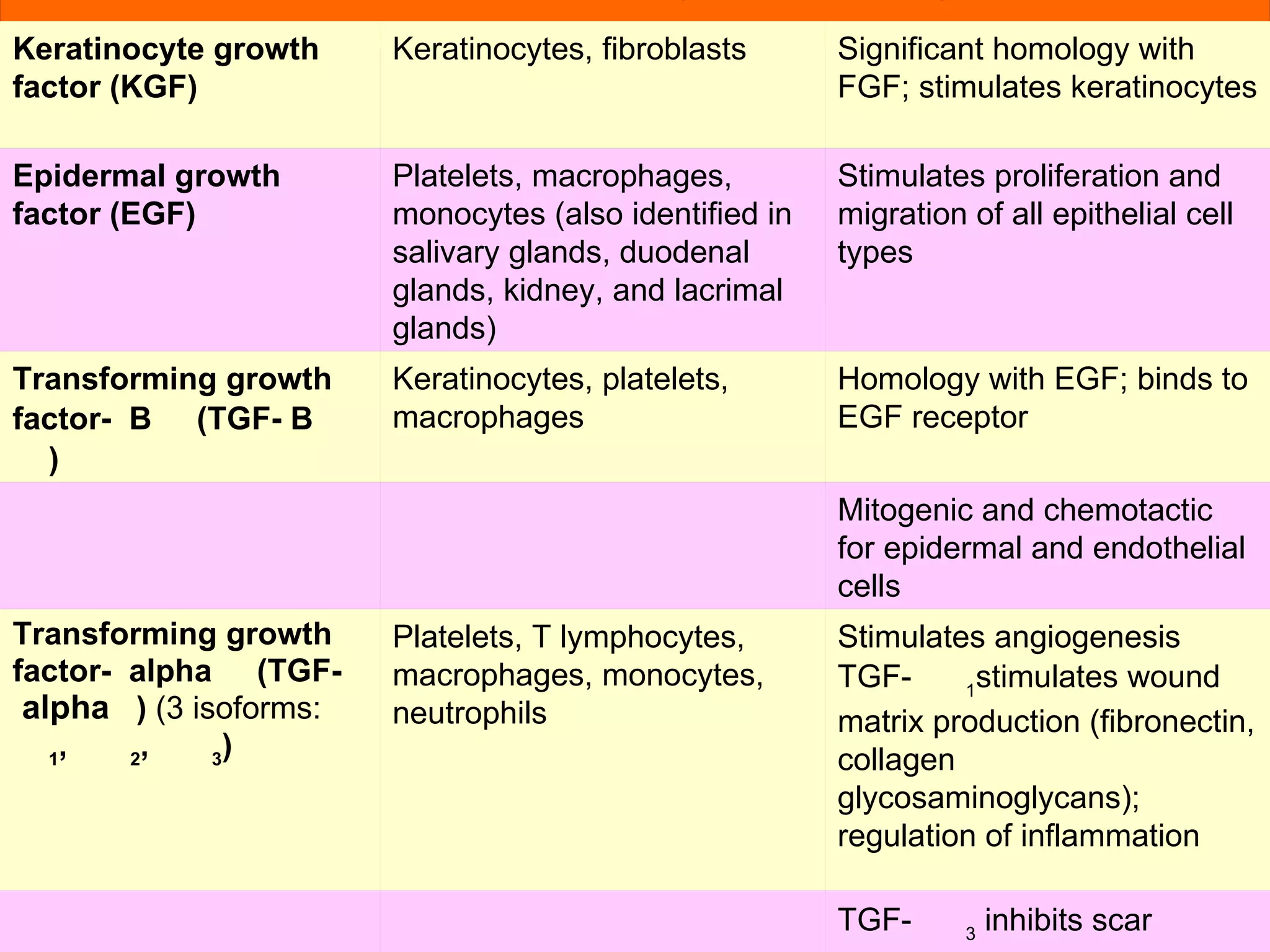
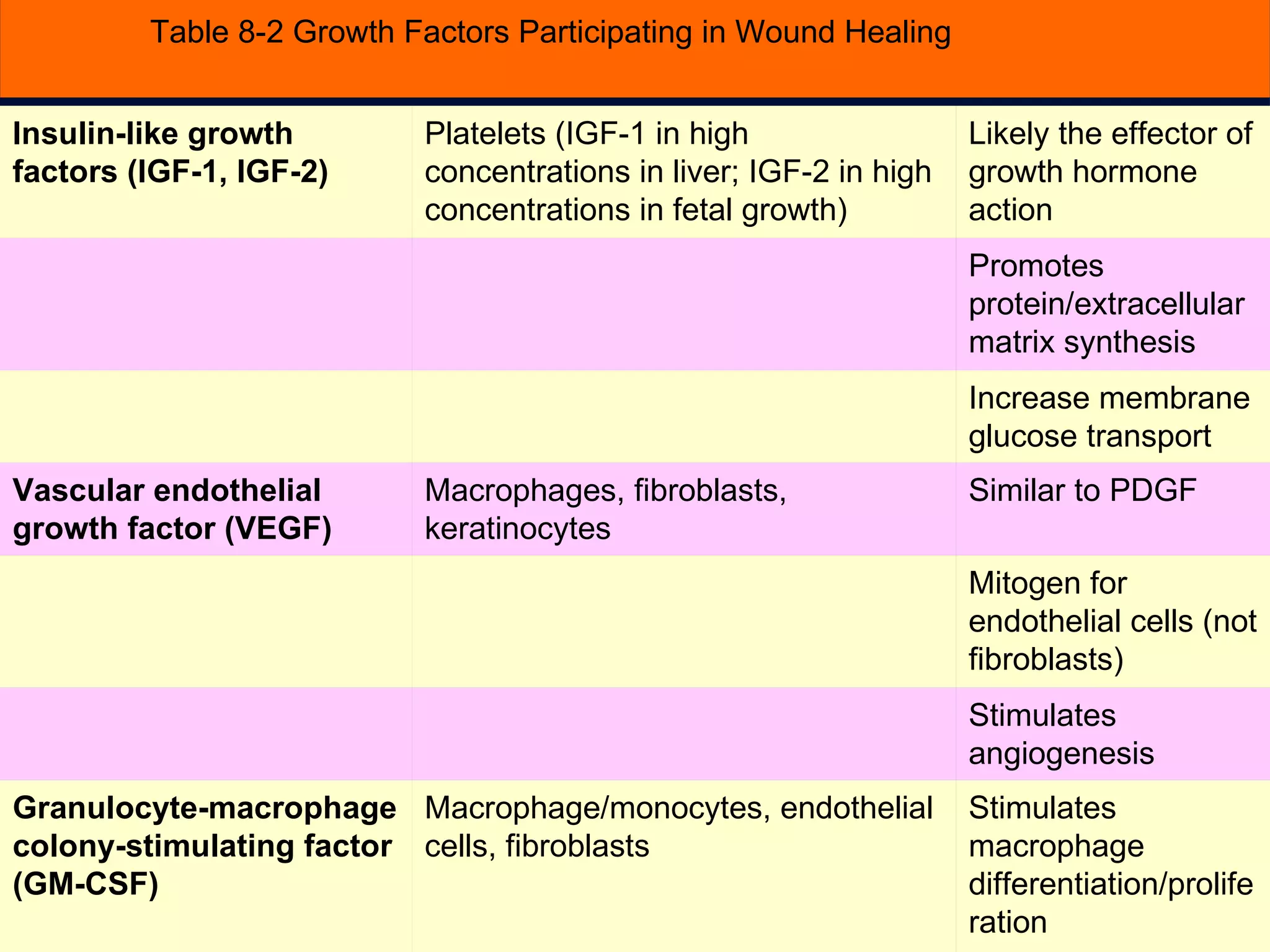
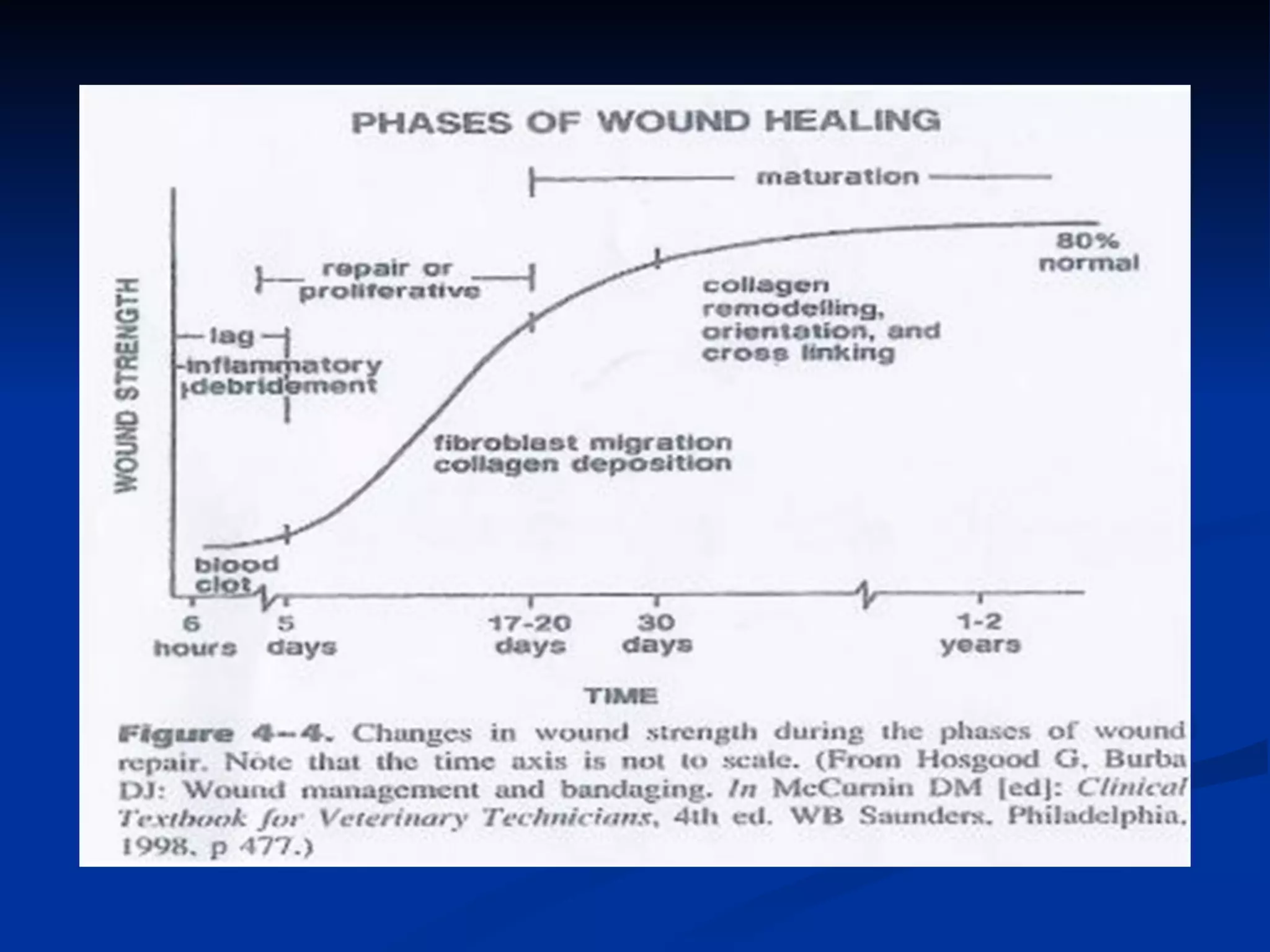
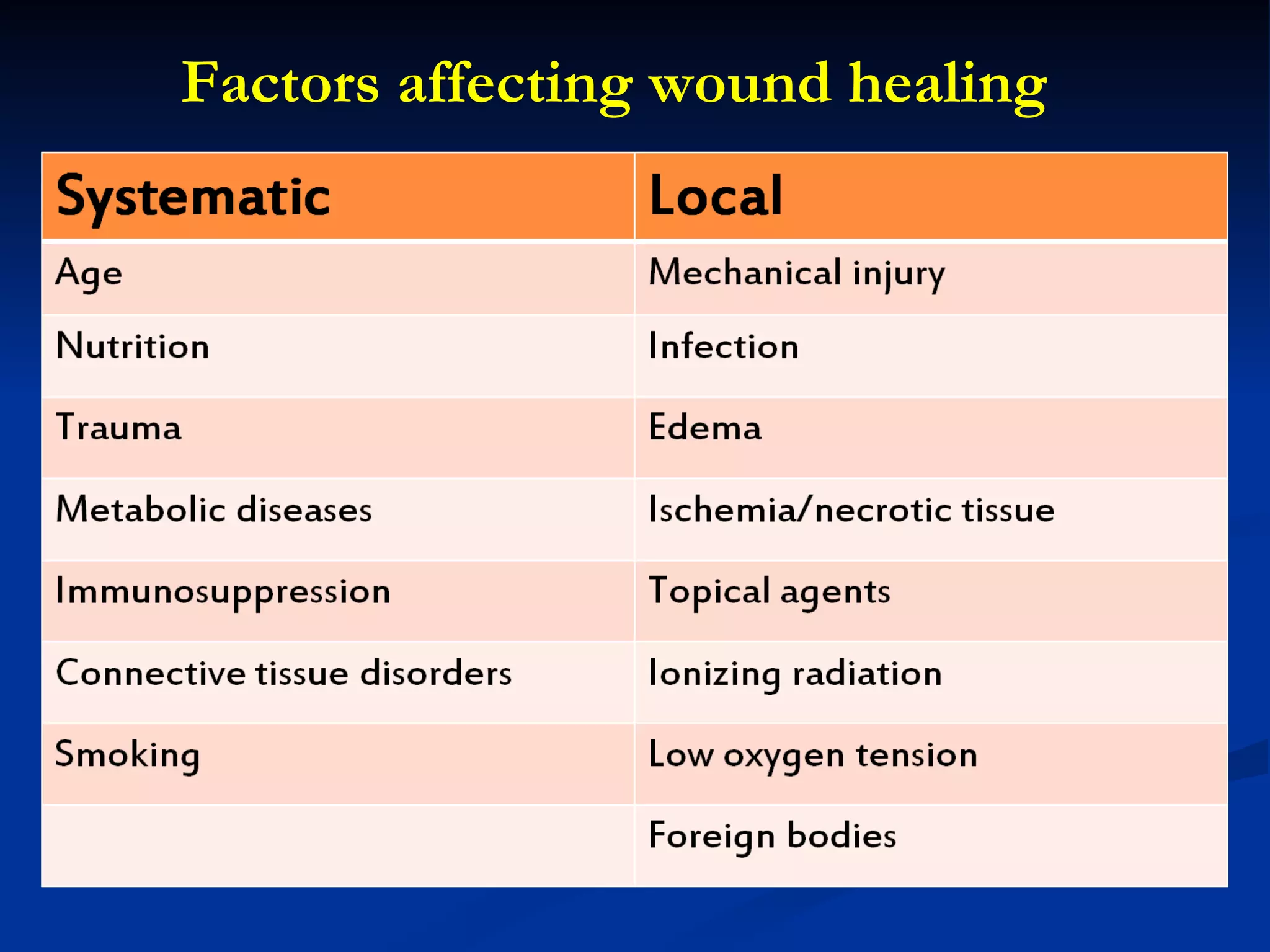
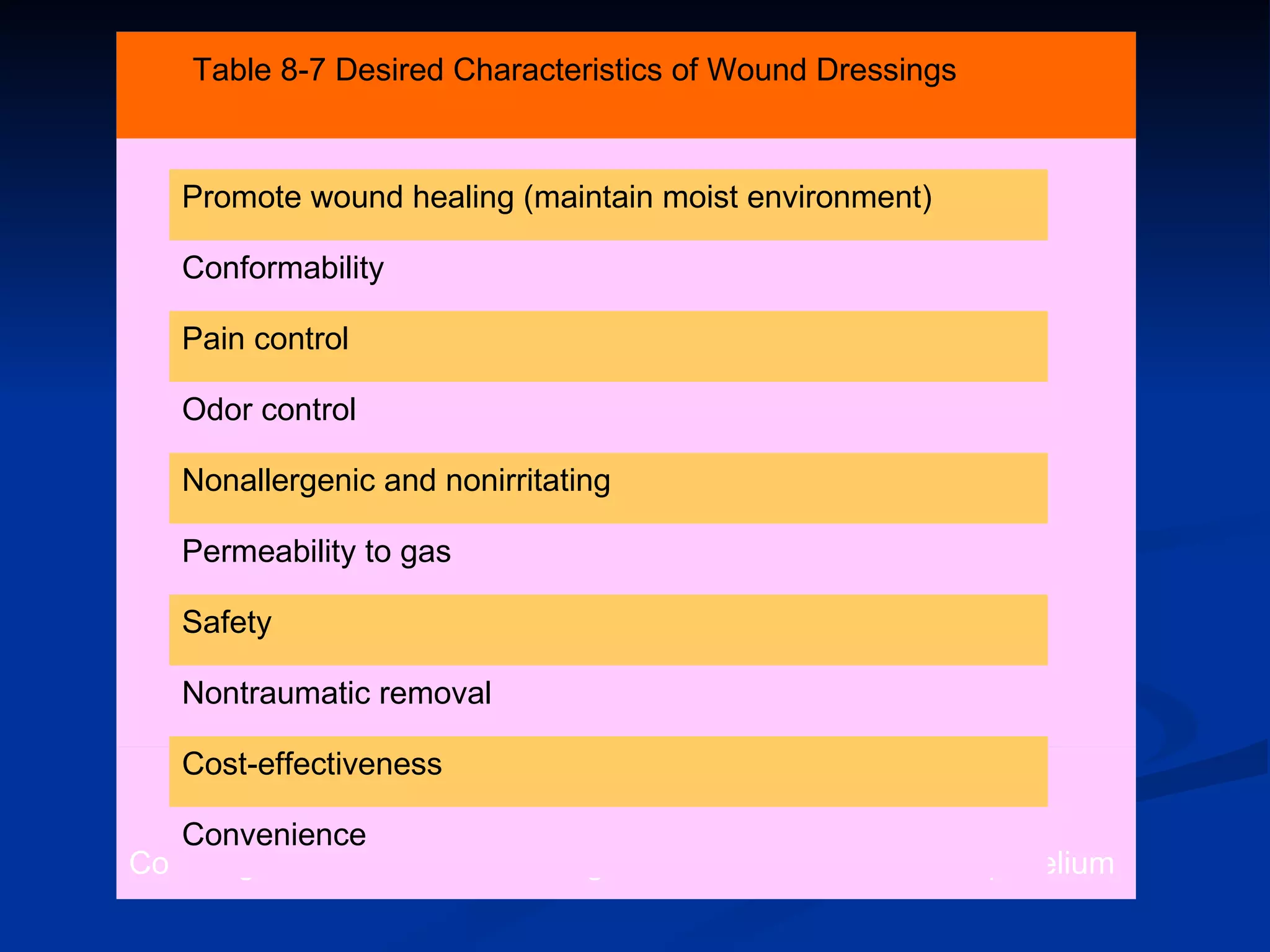
The document discusses the phases of wound healing: inflammatory, proliferative, and maturation. It describes key events in each phase such as angiogenesis, granulation tissue formation, epithelialization, collagen deposition, and scar remodeling. Growth factors and cytokines that participate in wound healing are also outlined.