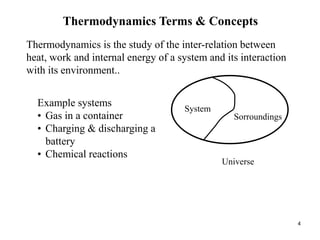
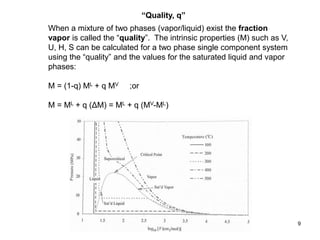
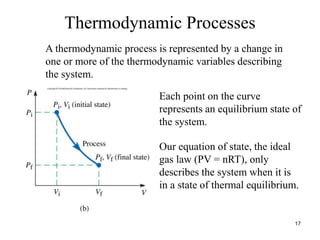
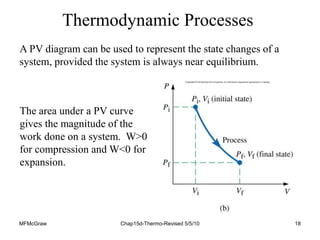
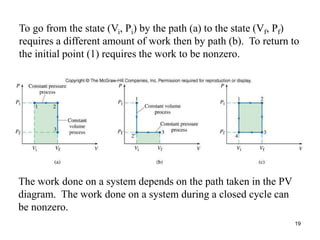
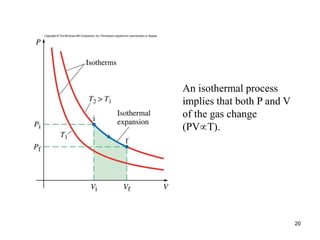
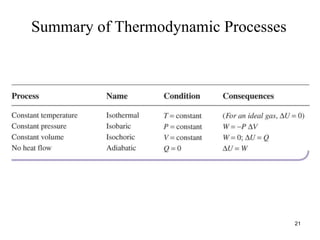
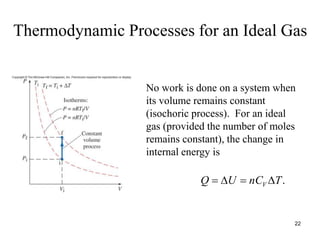
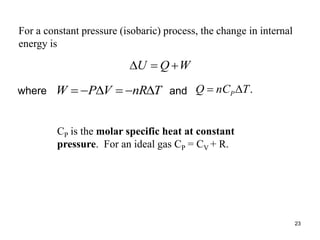
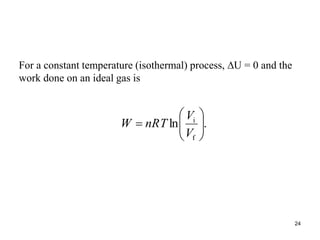
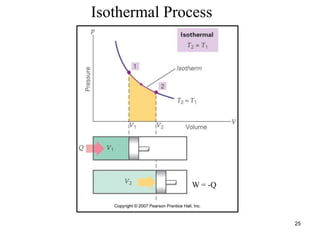
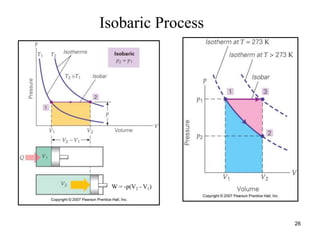
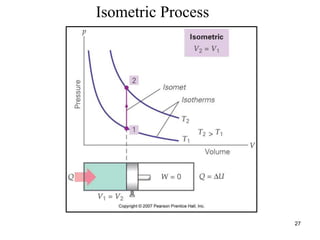
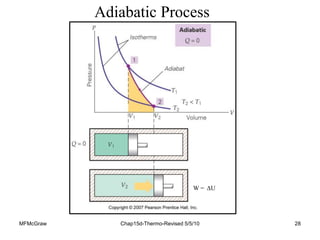
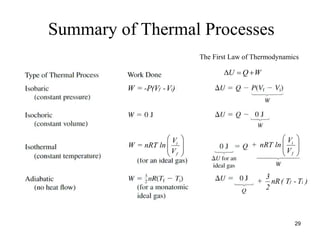
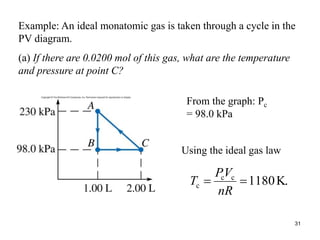
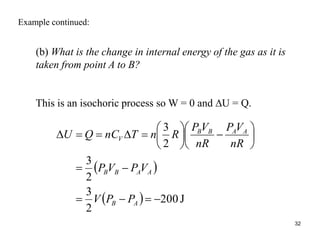
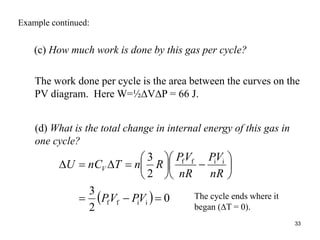
Thermodynamics I is a course on the fundamentals of thermodynamics including common terms and concepts, fluid properties, processes like isothermal and adiabatic, the laws of thermodynamics, heat engines and refrigeration. The course will cover these topics through lectures, recommended textbooks, videos and assessments including assignments, a test and exam. Students will learn about systems, surroundings, energy, work, heat, entropy and more. Properties of gases can be determined using equations of state from ideal gas laws, tables and diagrams.