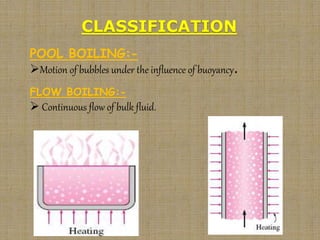
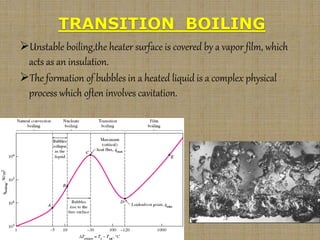
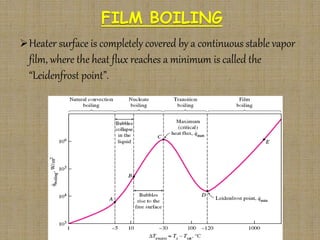
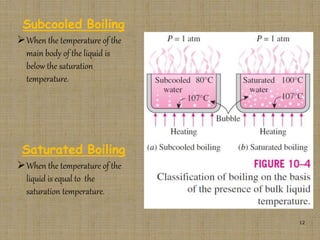
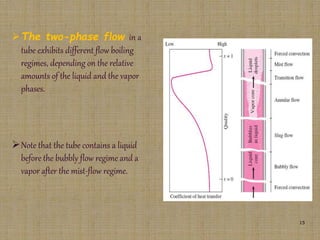
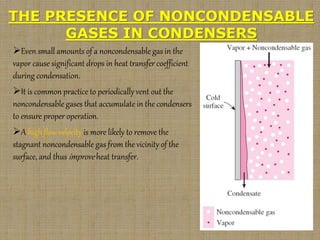
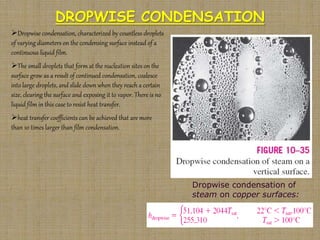
This document discusses boiling and condensation processes. It defines boiling as a liquid to vapor phase change and condensation as a vapor to liquid phase change. The document describes different types of boiling including nucleate, critical heat flux, transition, and film boiling. It also discusses pool boiling and flow boiling. For condensation, it covers film condensation and dropwise condensation. The key applications of boiling and condensation are in heat exchangers and refrigeration systems.