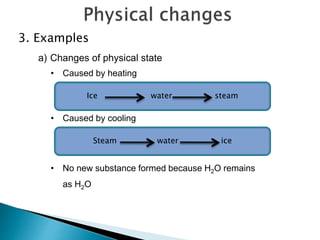
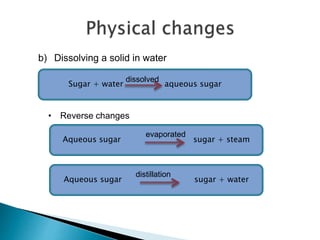
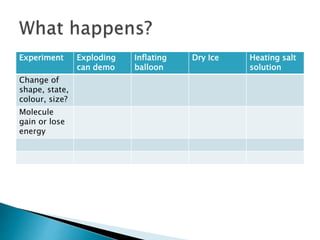
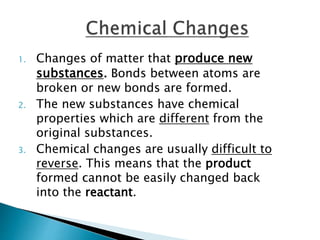
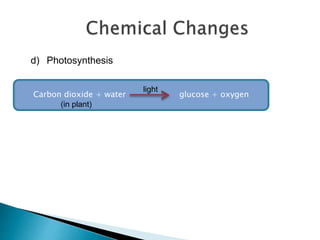
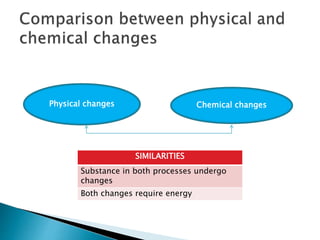
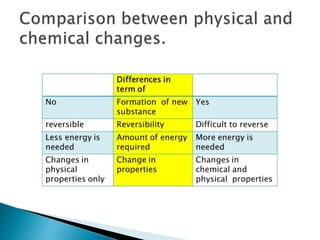
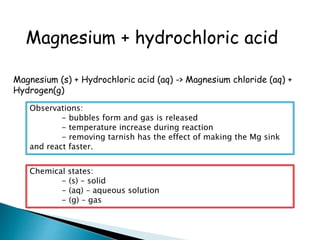
Physical changes alter a substance's physical properties but do not change its chemical composition. Chemical changes produce new substances with different chemical properties. A physical change is usually reversible while a chemical change produces new substances that cannot easily be reversed back to the original ones. Some examples of physical changes include changes of state from solid to liquid to gas, dissolving, and changes in shape or size. Chemical changes include burning, rusting, and cooking.