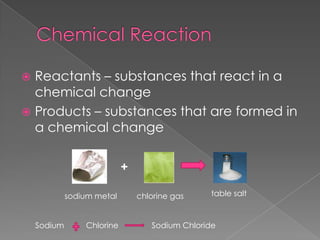
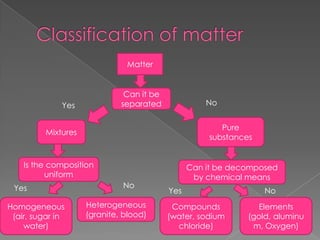
This document discusses the properties and changes of matter. It defines matter as anything that has mass and takes up space. It distinguishes between physical and chemical properties and changes. Physical changes do not alter the chemical identity of a substance, while chemical changes produce new substances. The three common states of matter are solids, liquids, and gases. Pure substances have a uniform composition and properties, while mixtures maintain the identities of their components but are not uniform throughout.