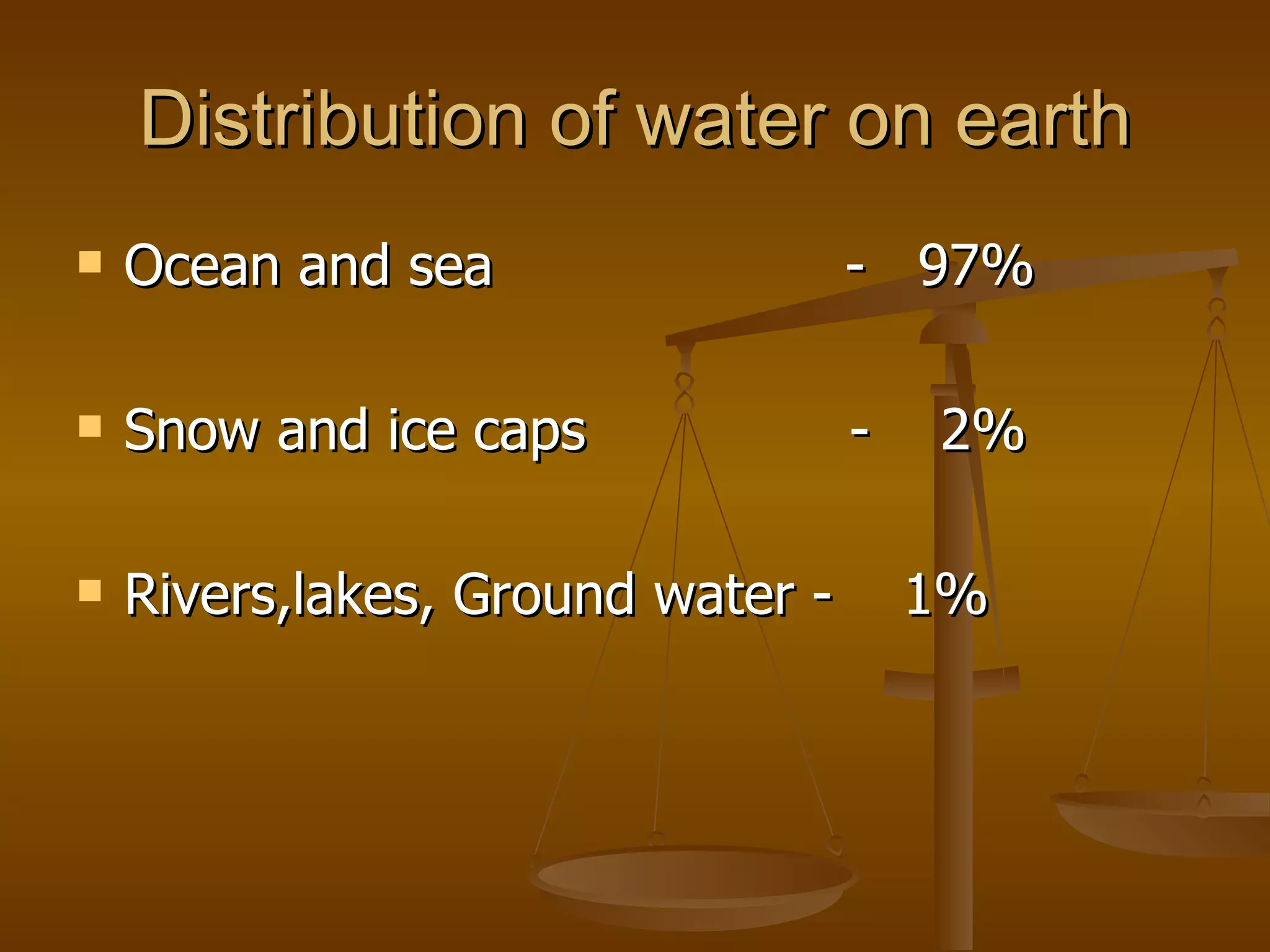
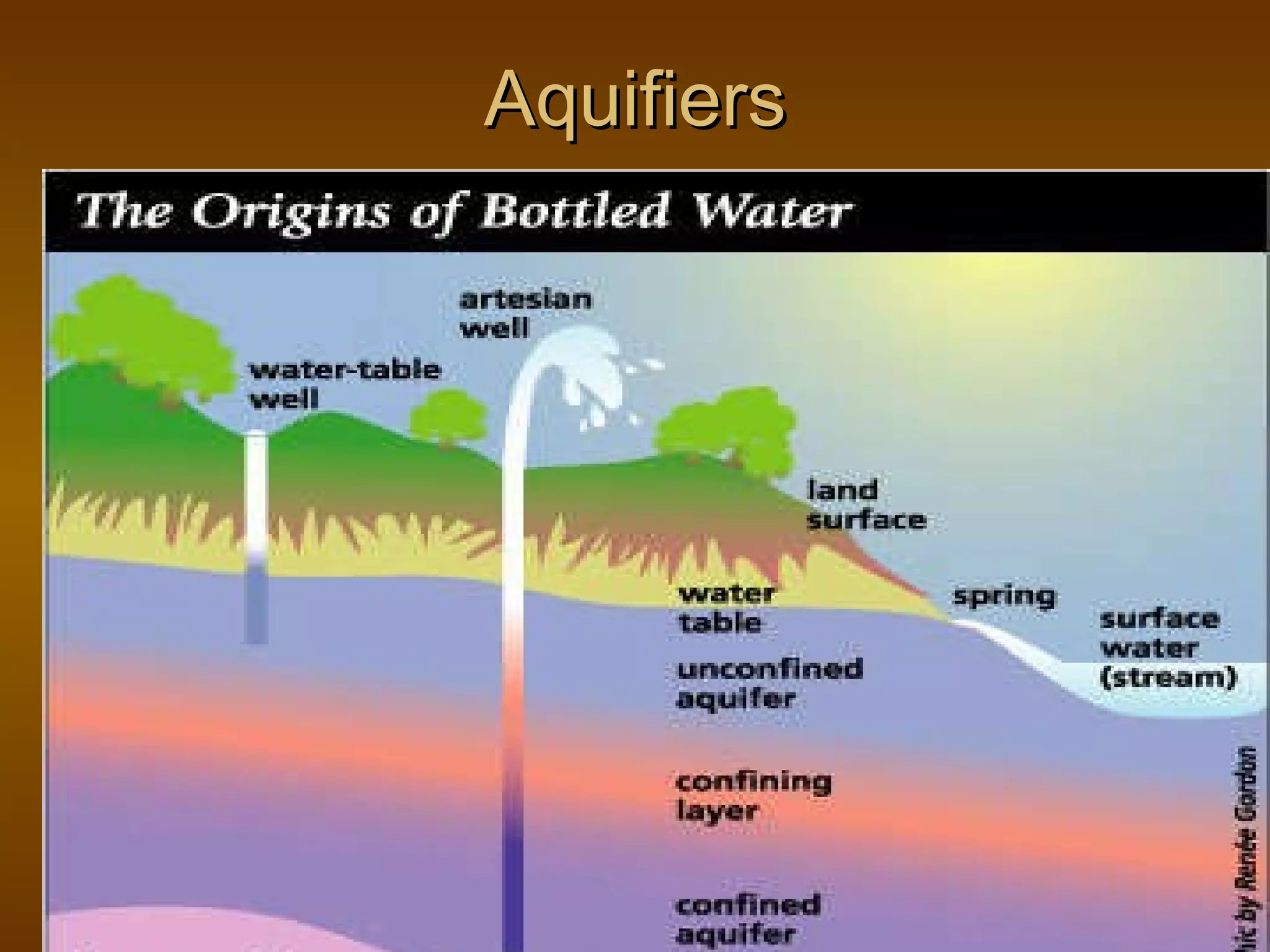
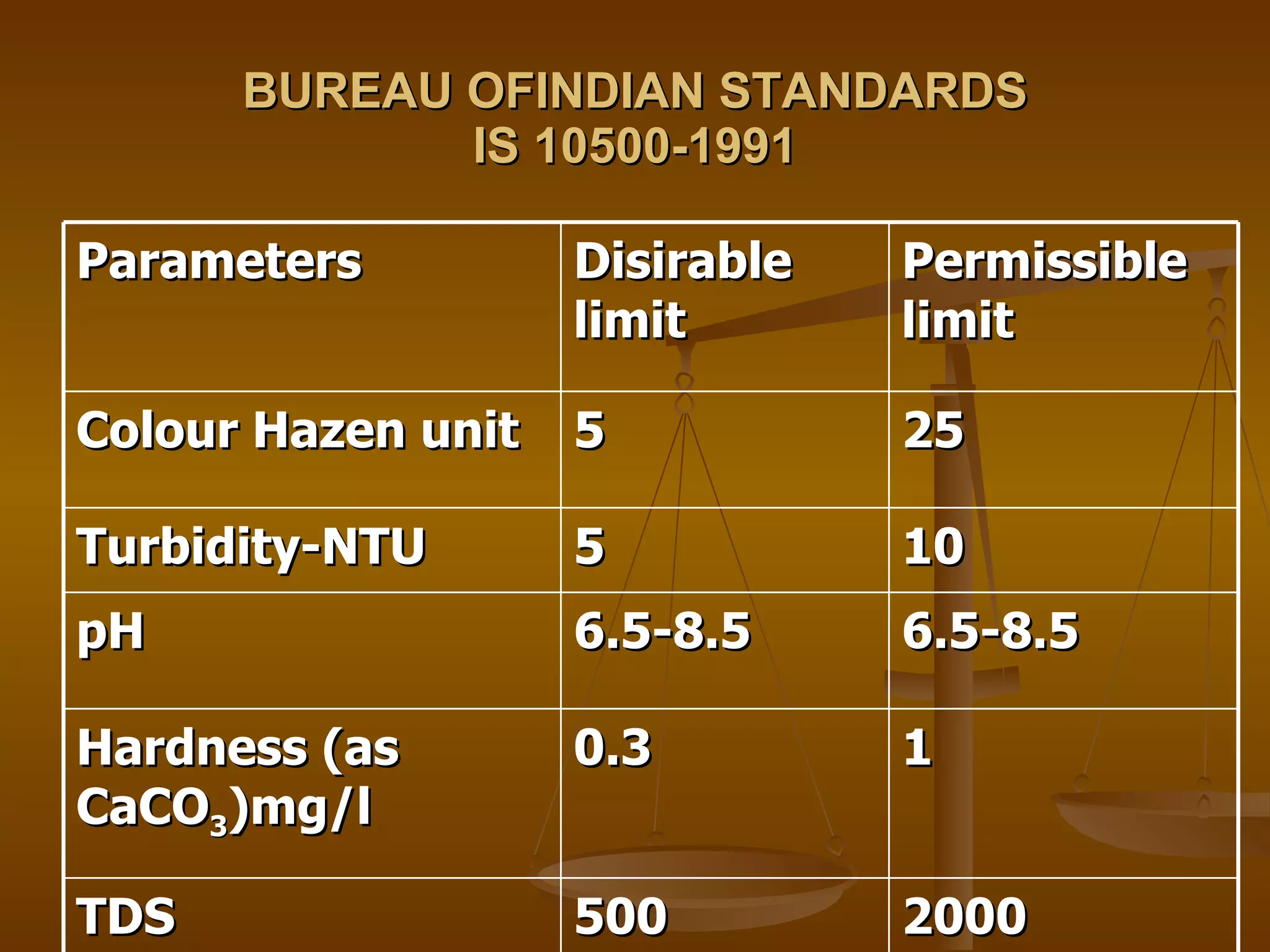
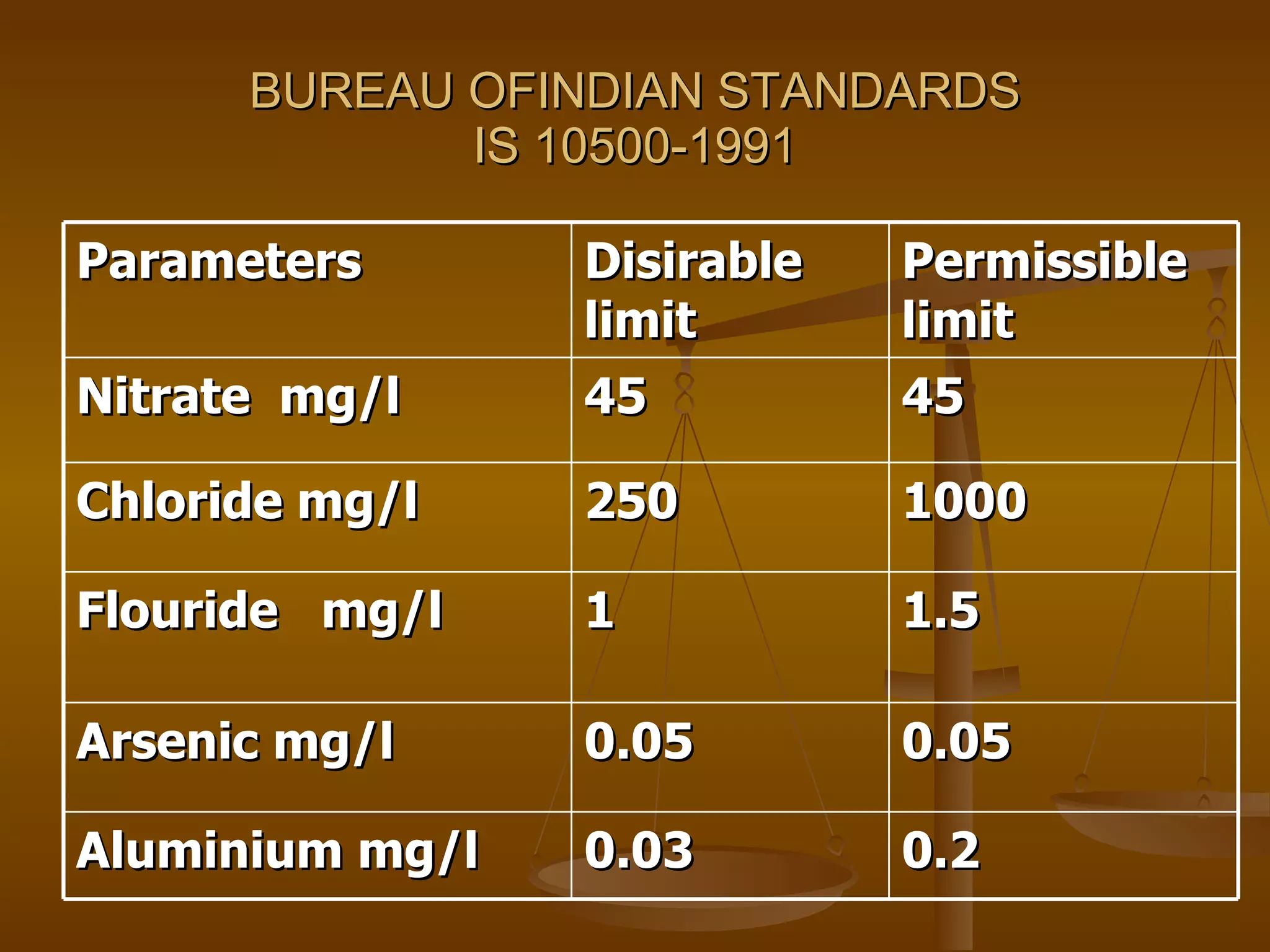
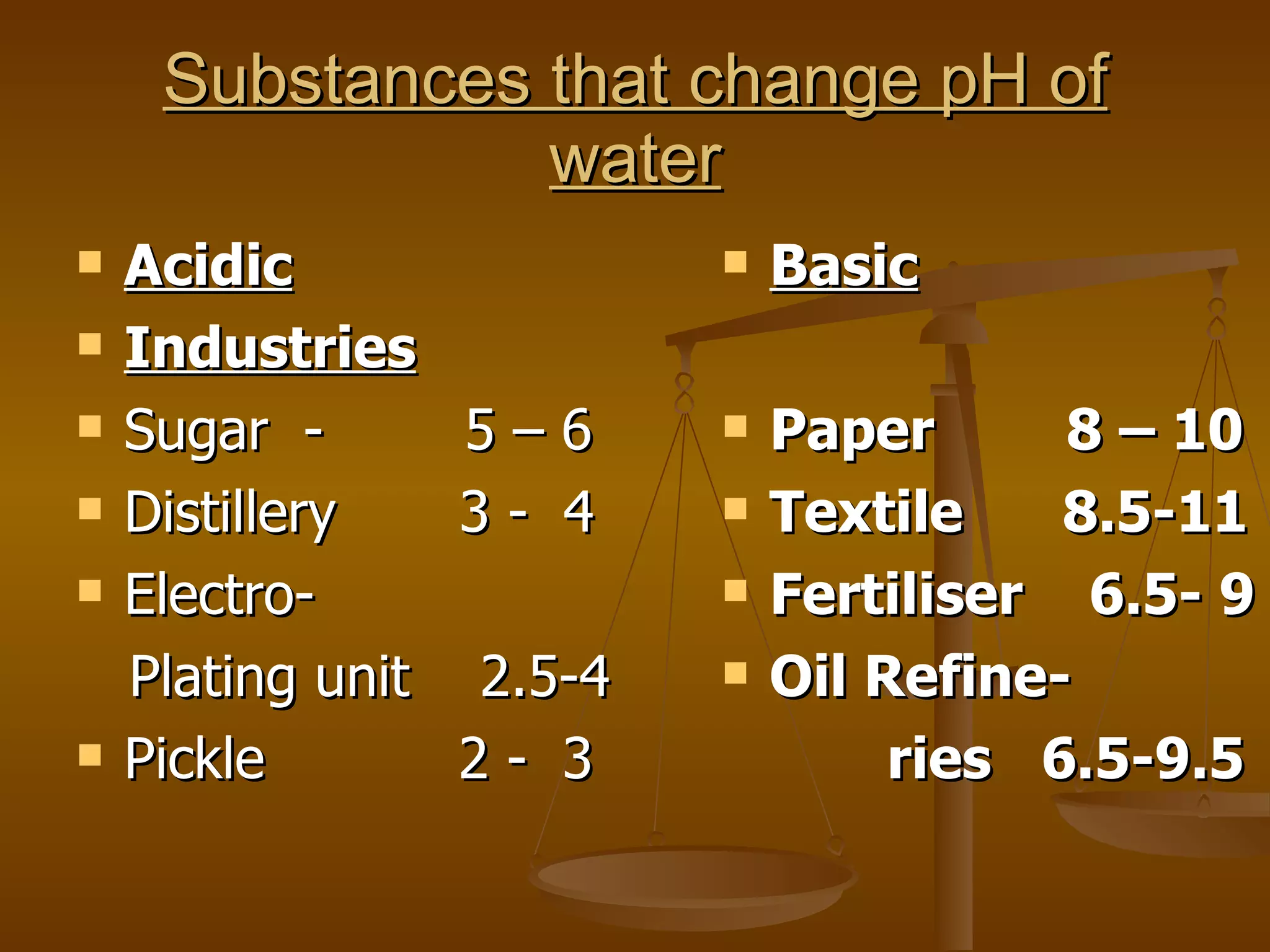
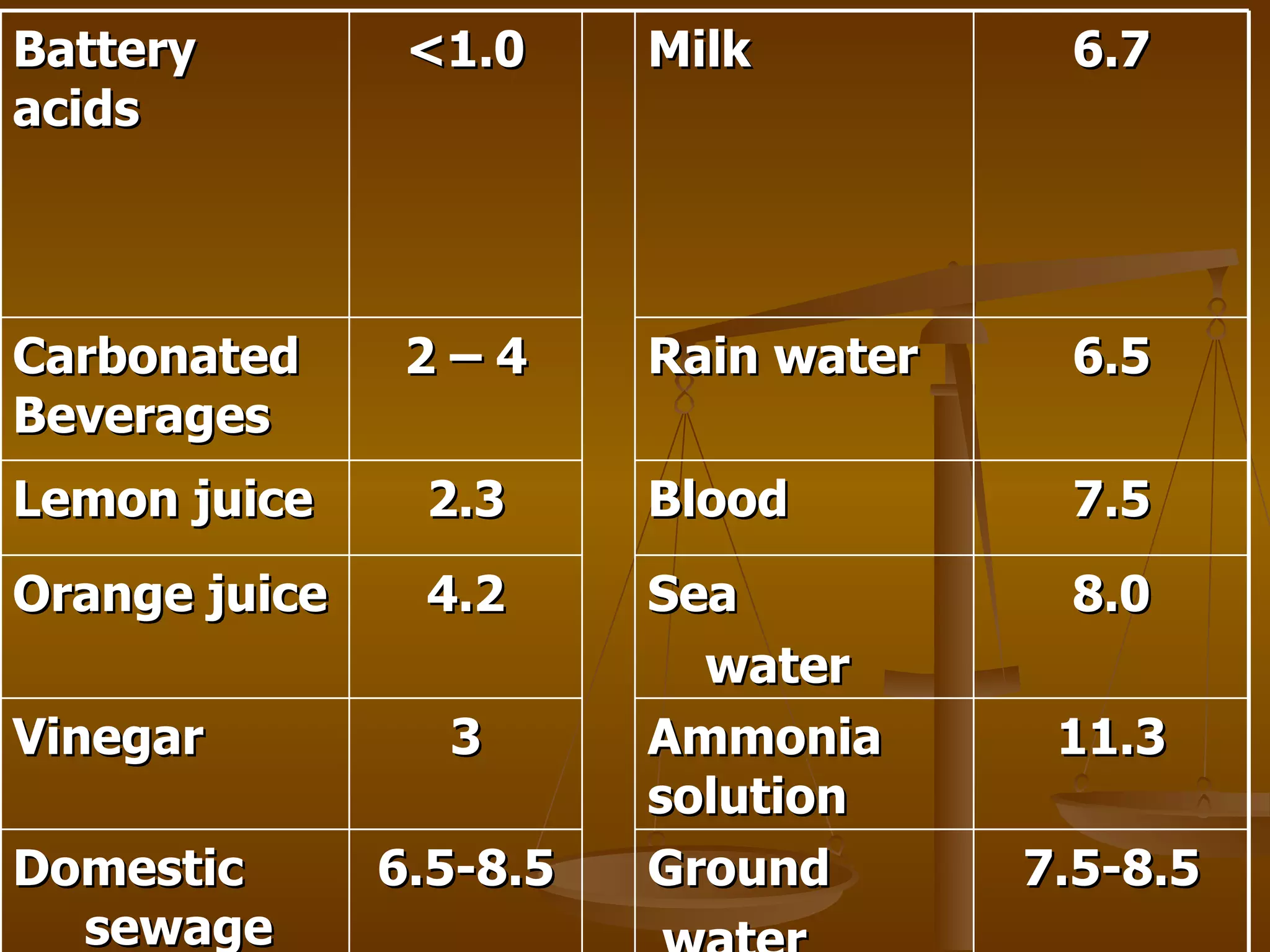
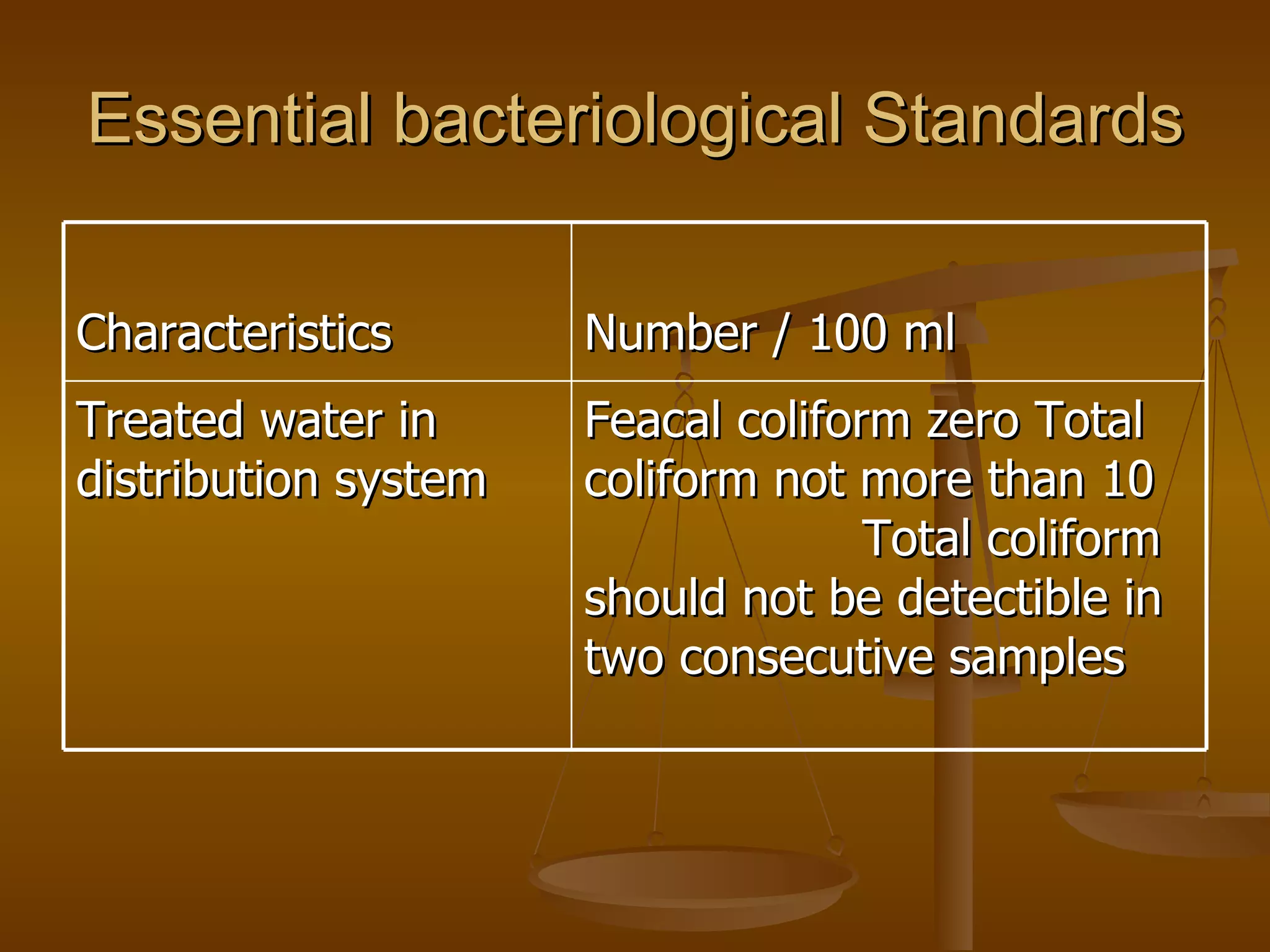
The document discusses various water quality parameters and standards. It outlines physical, chemical and bacteriological contaminants that can affect water quality. It provides desirable and permissible limits for several water quality parameters according to the Bureau of Indian Standards including color, turbidity, pH, hardness, chloride, nitrate and fluoride levels. It also discusses problems that can arise due to contamination and failures to meet water quality standards.