Embed presentation
Downloaded 97 times



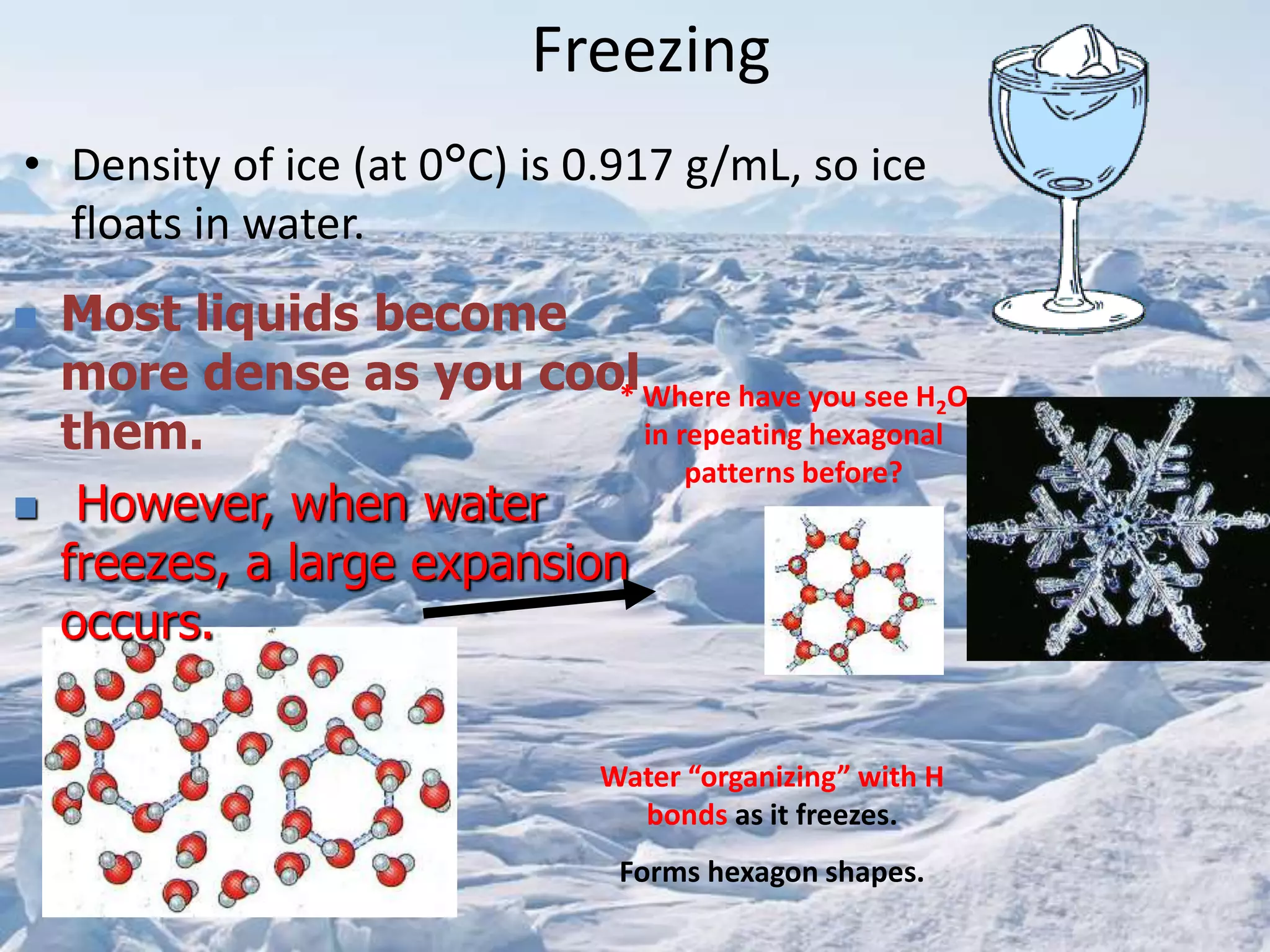
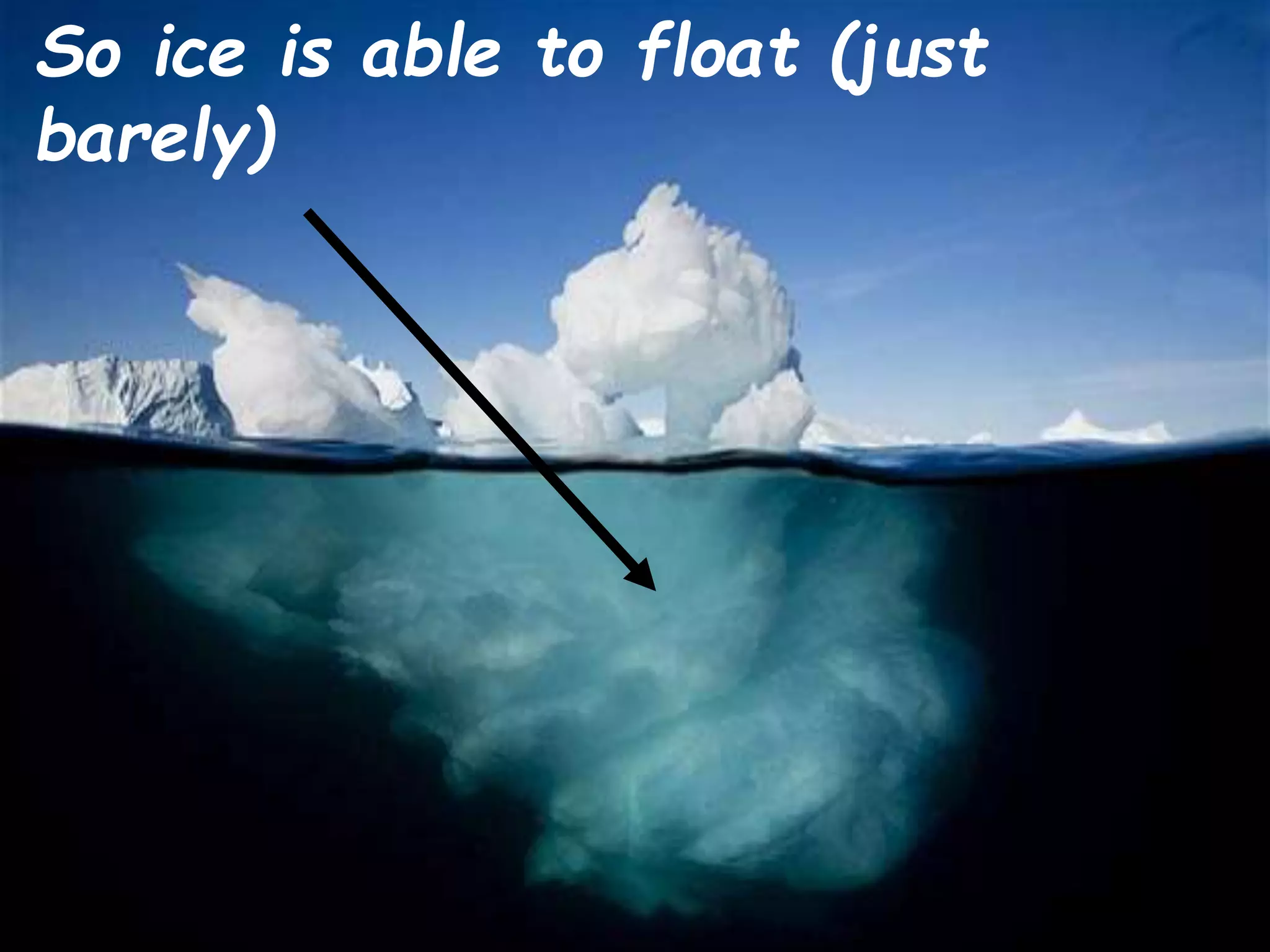



Water has unique physical properties. It is transparent, colorless, odorless and tasteless in its pure form. At normal atmospheric pressure, water boils at 100°C. When water freezes at 0°C, it expands and forms hexagonal structures as ice, allowing ice to float on liquid water. Pure water is not a good conductor of electricity, but conductivity increases as the concentration of dissolved ions increases. Water is also a remarkable solvent that can dissolve many substances to form aqueous solutions because of its high dielectric constant.







