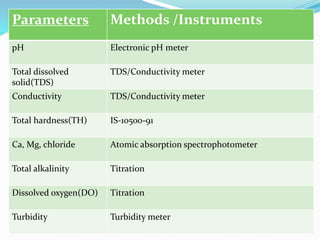
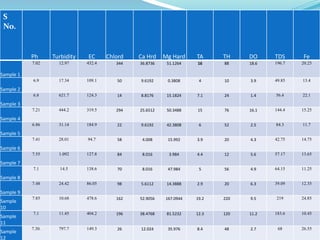
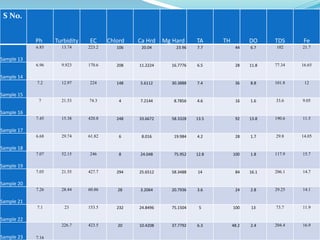
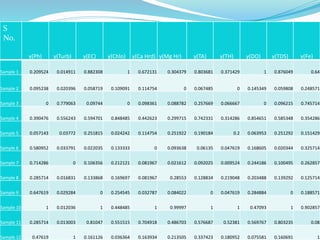
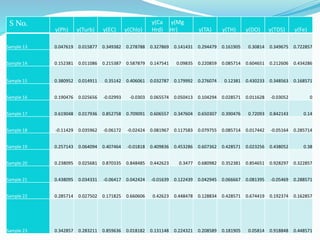
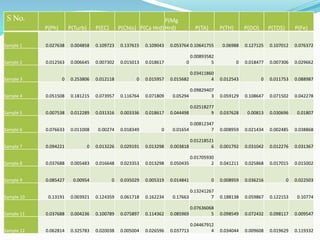
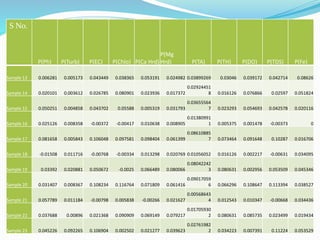
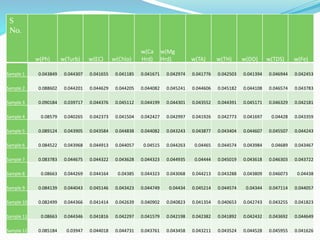
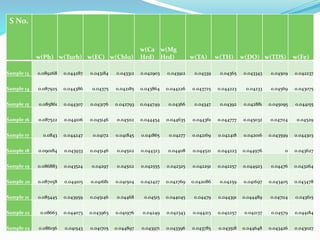
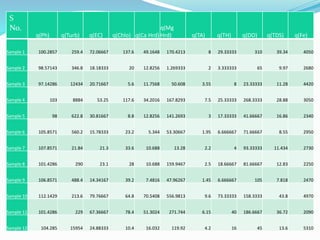
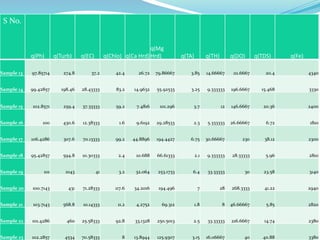
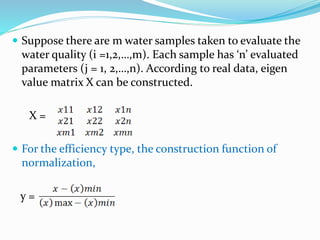
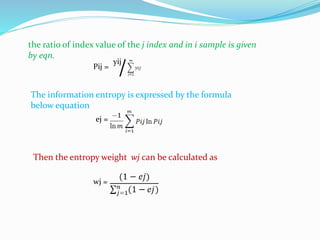
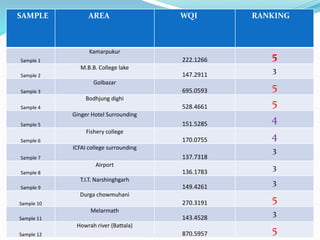
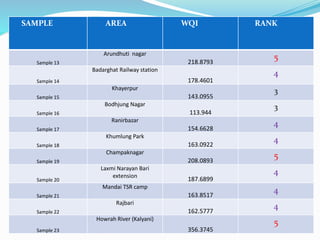
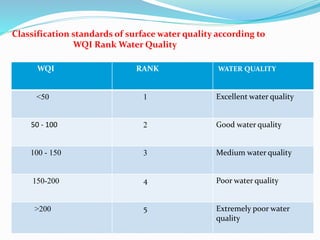
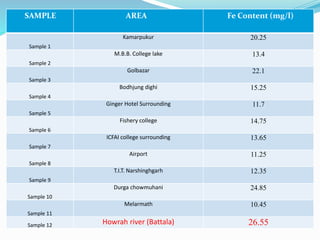
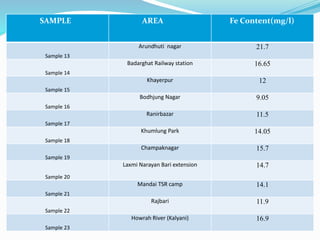
The document discusses the analysis of surface water quality in Tripura, India. 23 surface water samples were collected from various locations and analyzed for parameters like pH, turbidity, conductivity, calcium, magnesium, iron and more. The water quality index was determined using the concept of information entropy to check if the water was suitable for drinking. The principles, methods, equipment and procedures for determining various physico-chemical parameters of the water samples are also outlined.