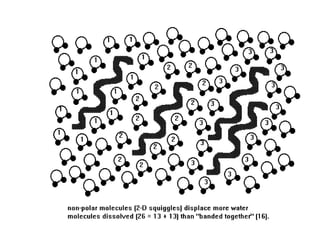
Hydrogen bonds between water molecules give water many unique properties, including:
1. Being a liquid at room temperature due to cohesion between molecules.
2. Having a high specific heat capacity because breaking hydrogen bonds requires energy.
3. Having a high heat of vaporization because it takes energy to break hydrogen bonds during evaporation.