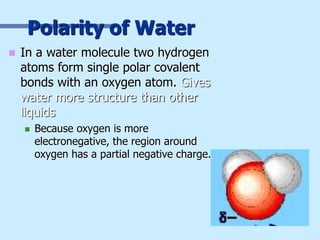
Water has unusual properties due to its polarity and ability to form hydrogen bonds between molecules. Its polarity allows water to serve as a universal solvent and exhibit properties like surface tension, capillary action, and a high heat of vaporization. The hydrogen bonds give water a high heat capacity and allow ice to float, and are responsible for many of water's unique physical behaviors important for life.