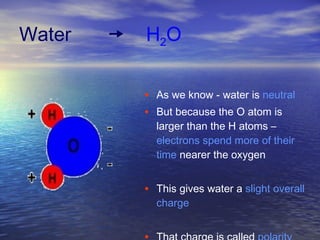
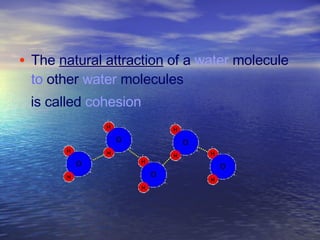
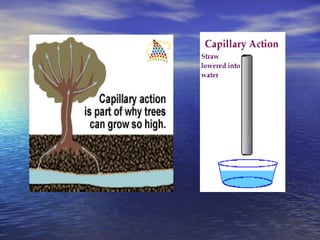
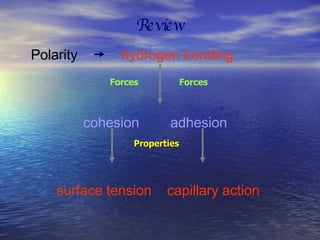
Water has unique properties primarily due to its polarity. Polarity arises because oxygen is more electronegative than hydrogen, giving water molecules an overall slight charge. This polarity allows water molecules to form hydrogen bonds, contributing to properties like cohesion, adhesion, surface tension, and capillary action. Water is the most abundant naturally occurring liquid on Earth and is unusual in that it expands when frozen.