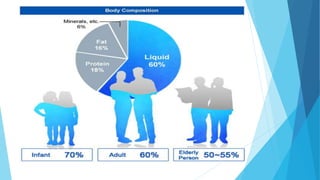
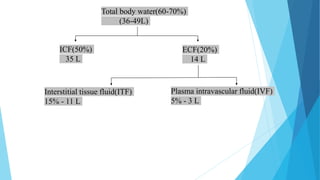
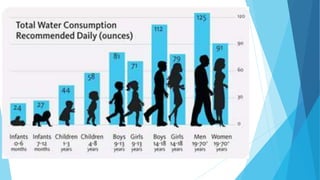
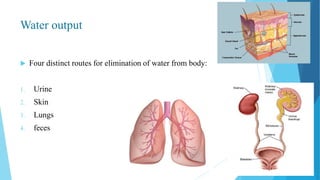
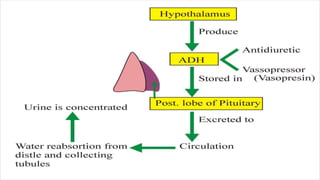
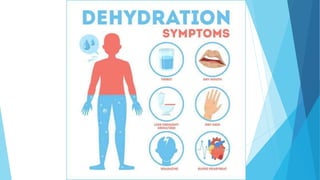
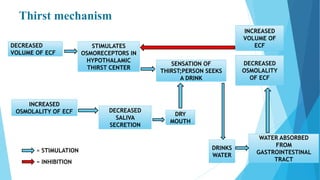
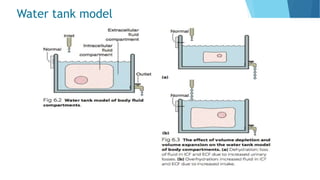
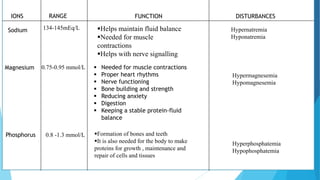
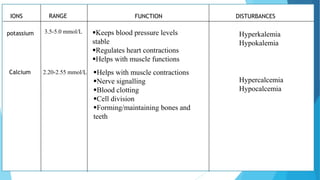
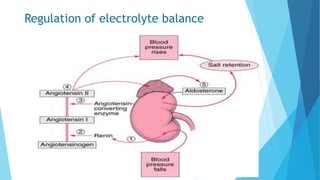
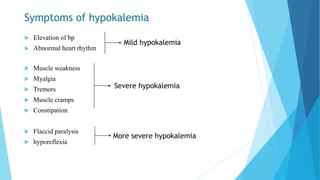
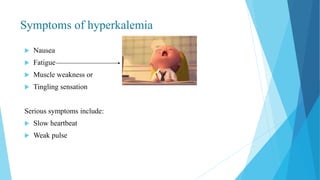
This document provides an overview of fluid and electrolyte balance in the human body. It discusses water intake and output, the distribution and regulation of body water, and hormonal control of fluid balance. Electrolyte composition, distribution, and regulation are described. Common electrolyte imbalances like hyponatremia, hypernatremia, hypokalemia, and their symptoms and treatments are summarized. The document emphasizes the importance of fluid and electrolyte homeostasis for normal physiological functioning.















































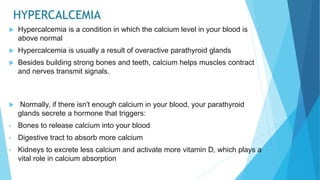











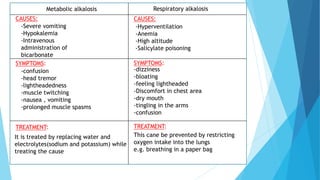
![Acid base balance
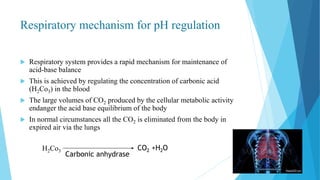
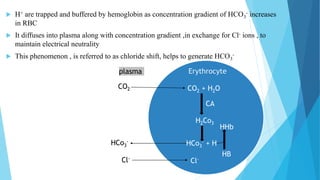
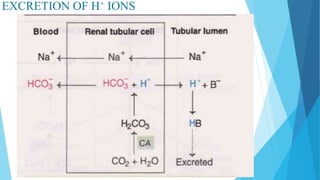
Acid base balance means regulation of [H+] in the body fluid
Even slight changes in H+ value from normal can cause marked
alteration in the rate of chemical reaction in the cells
For this reason the regulation of H+ is the most important aspect of
homeostasis](https://image.slidesharecdn.com/fluidandelectrolytebalance-210618182333/85/Fluid-and-electrolyte-balance-76-320.jpg)





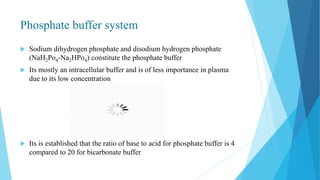
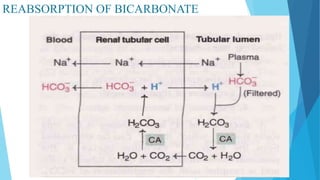
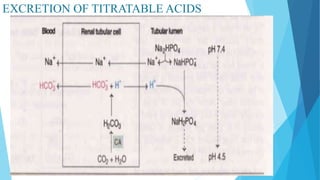
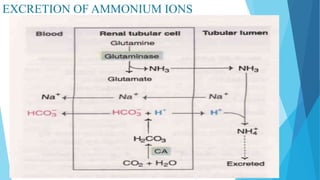
![Bicarbonate buffer system
Sodium bicarbonate and carbonic acid (NaHCO3-H2CO3) is the most predominant buffer
system of ECF , particularly the plasma
Carbonic acid dissociates into hydrogen and bicarbonate ions
Henderson- hasselbalch equation:
pH = pKa + log
It is evident from this equation that the pH is dependent on ratio of the concentration
of base to acid
[Base]
[Acid]](https://image.slidesharecdn.com/fluidandelectrolytebalance-210618182333/85/Fluid-and-electrolyte-balance-82-320.jpg)