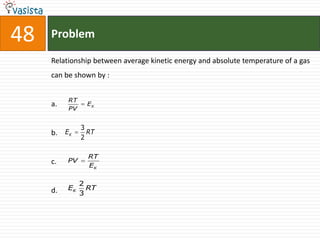
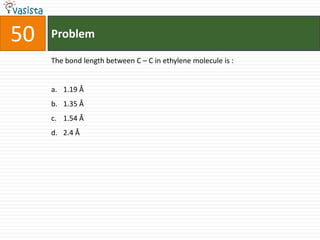
This document contains an unsolved chemistry paper from 2000 containing 50 multiple choice questions testing concepts such as:
1) Properties and reactions of acids, bases, salts, and other chemicals
2) Gas laws, thermodynamics, equilibrium, and stoichiometry
3) Structure and bonding in organic and inorganic compounds
4) Characteristics and trends in the periodic table
5) Techniques for separating and purifying compounds
The questions cover a wide range of fundamental chemistry topics and are intended to evaluate students' understanding of core concepts through multiple choice responses.







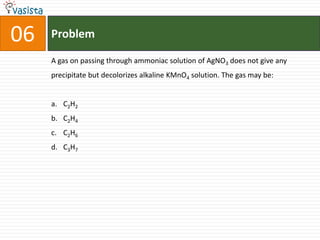


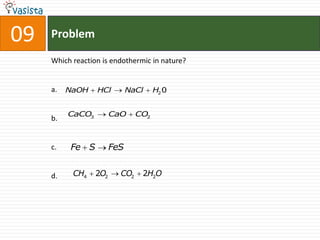


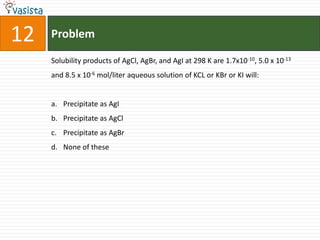
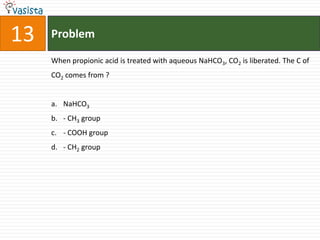
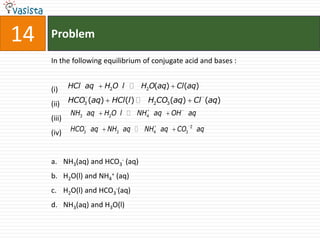

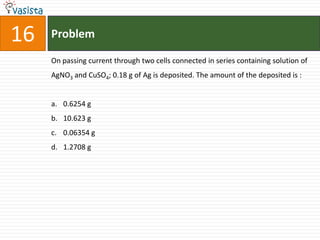









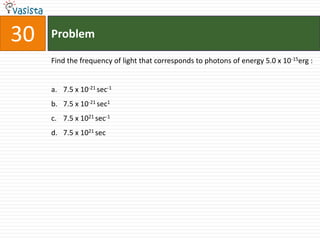







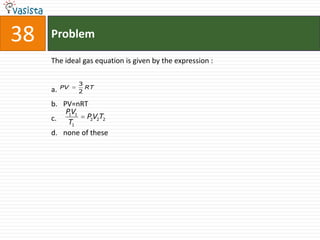

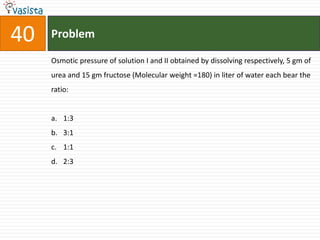


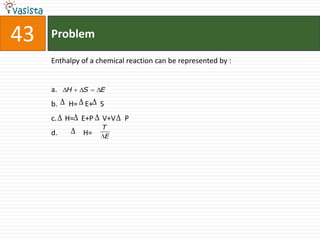
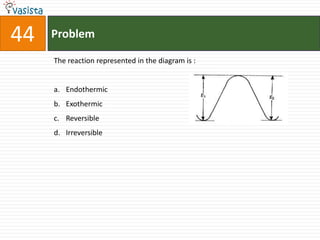
![45 Problem
In a reaction, the rate expression is, rate=K[A][B]2/3. The order of reaction is:
a. Zero
b. 1
c. 2
d. 5/3](https://image.slidesharecdn.com/chemistry-2000-120103060931-phpapp01/85/AMU-Chemistry-2000-47-320.jpg)