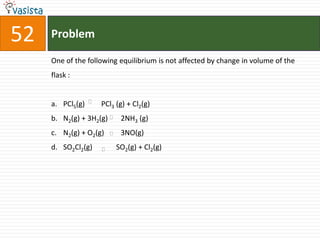
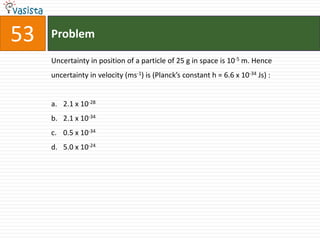
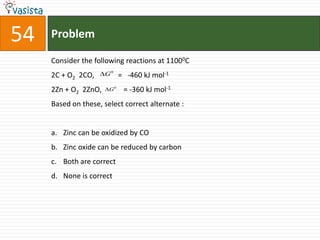
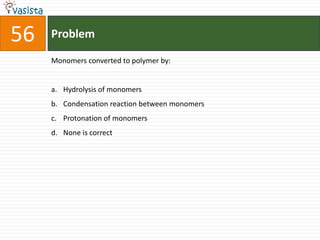
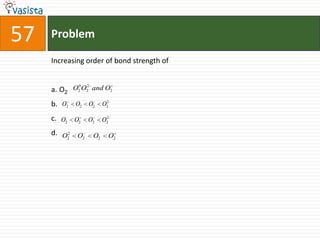
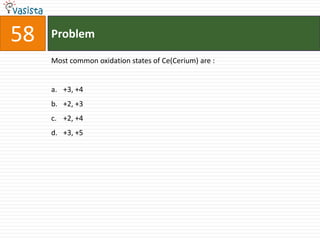
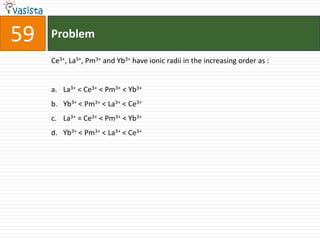
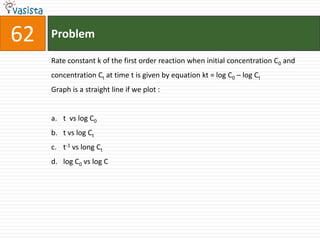
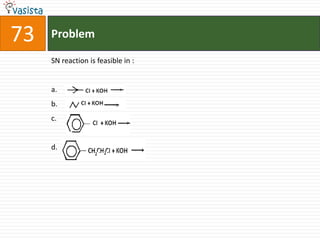
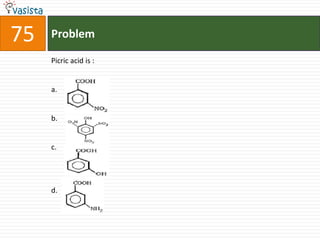
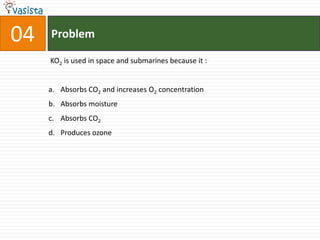
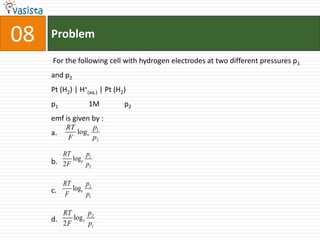
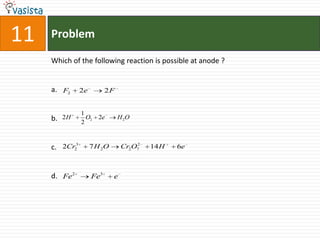
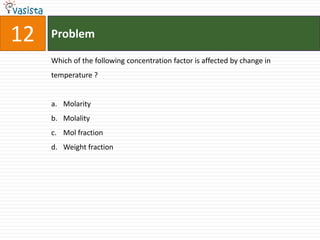
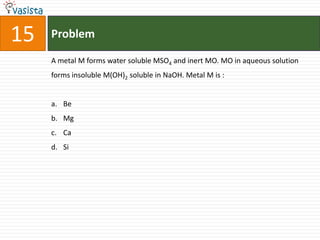
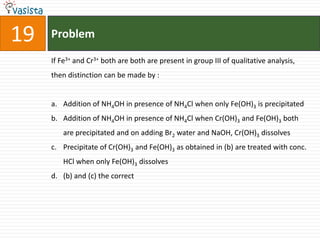
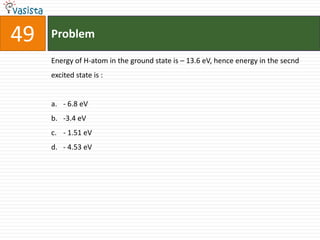
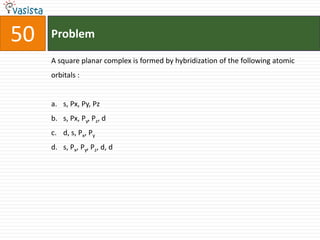
This document contains a chemistry exam with multiple choice questions. It tests concepts related to redox reactions, gas laws, organic chemistry reactions, transition metals, acid-base chemistry, and more. There are 65 total questions with 4 possible answers for each question labeled A, B, C, or D. The questions cover a wide range of chemistry topics and concepts at an undergraduate level.


![Problem01Which of the following is a redox reaction ? NaCl + KNO3 NaNO3 + KClCaC2O4 + 2HCl CaCl2 + H2C2O4Ca(OH)2 + 2NH4Cl CaCl2 + 2NH3 + 2H2O2K[Ag(CN)2] + Zn 2Ag + K2 [Zn(CN)4]](https://image.slidesharecdn.com/chemistry-2002-110924045106-phpapp02/85/AIEEE-Chemistry-2002-3-320.jpg)










![Problem17The most stable ion is : [Fe(OH)5]3-[FeCl6]3-[Fe(CN)6]3-[Fe(H2O)6]3+](https://image.slidesharecdn.com/chemistry-2002-110924045106-phpapp02/85/AIEEE-Chemistry-2002-19-320.jpg)

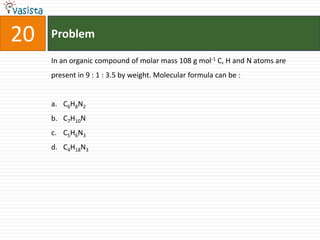
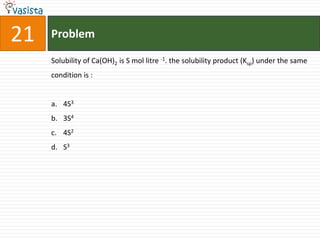
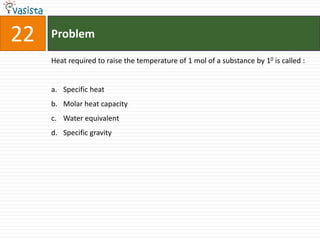

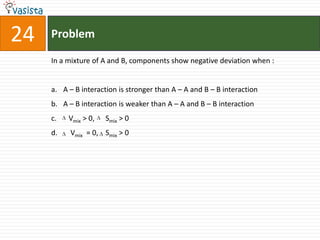
















![Problem51Type of isomerism shown by [Cr(NH3)5NO2]Cl2 is : Optical Ionization Geometrical Linkage](https://image.slidesharecdn.com/chemistry-2002-110924045106-phpapp02/85/AIEEE-Chemistry-2002-53-320.jpg)