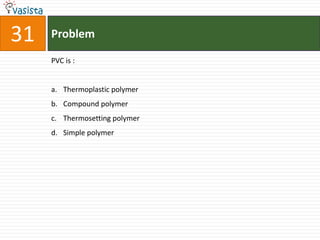
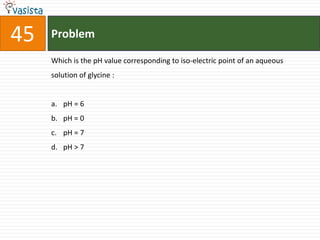
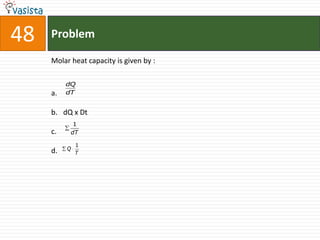
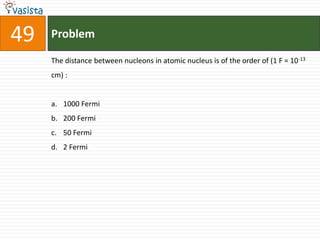
This document contains an unsolved chemistry exam from 1998 containing 50 multiple choice questions testing knowledge of chemistry concepts and principles. The questions cover topics including chemical compounds and reactions, periodic table properties, organic chemistry, polymers, and environmental chemistry. An answer key is provided to check solutions.