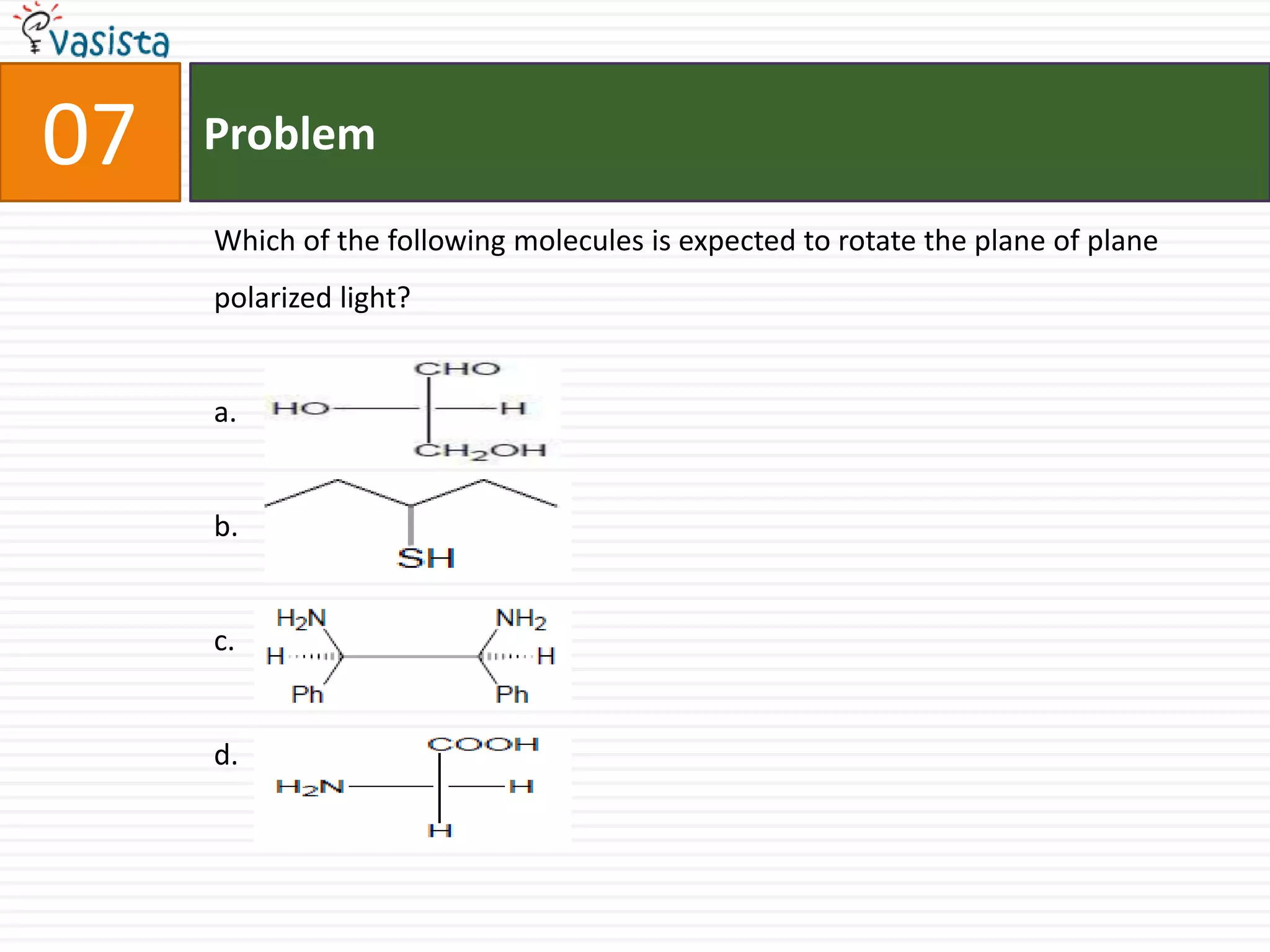
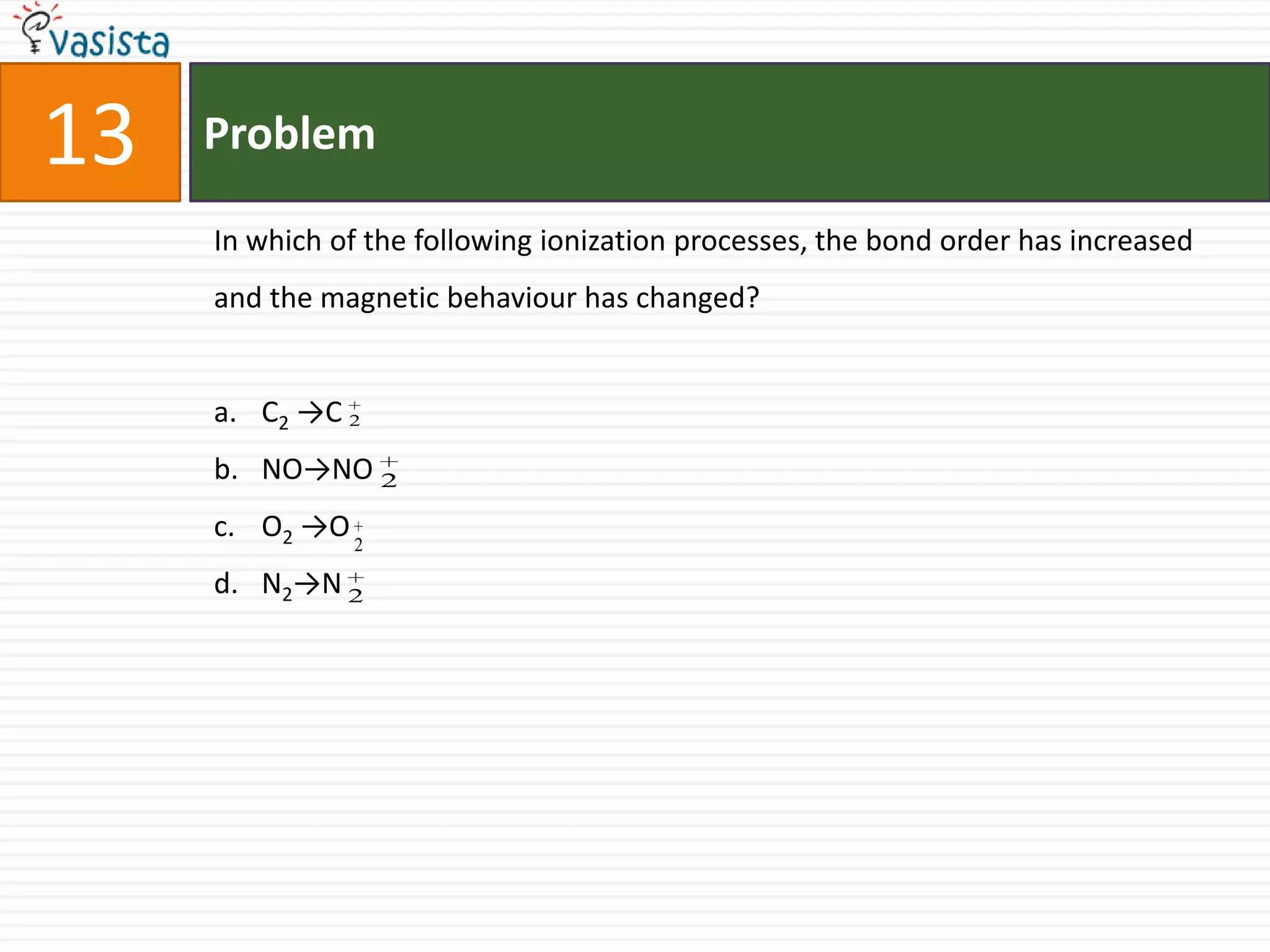
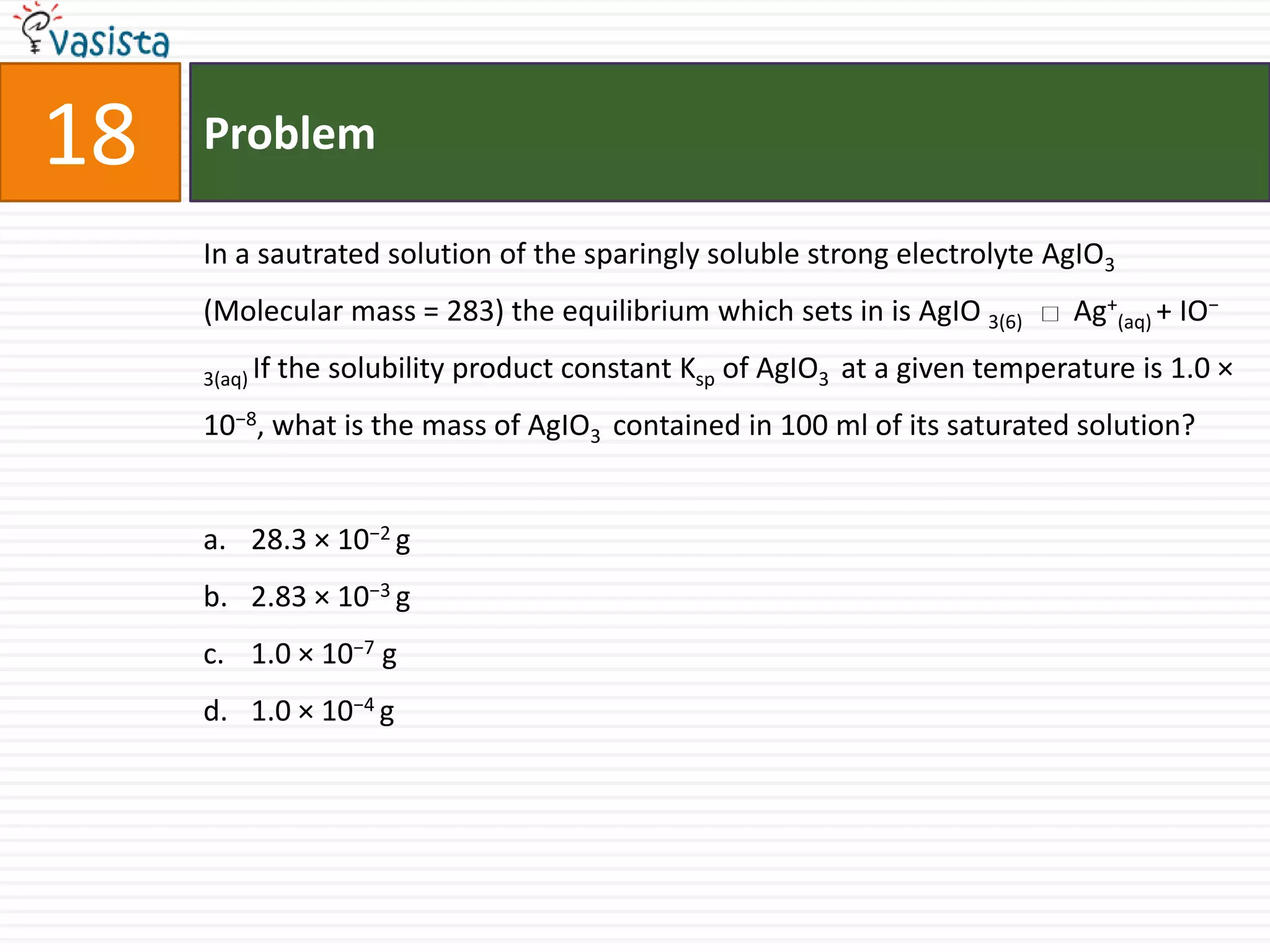
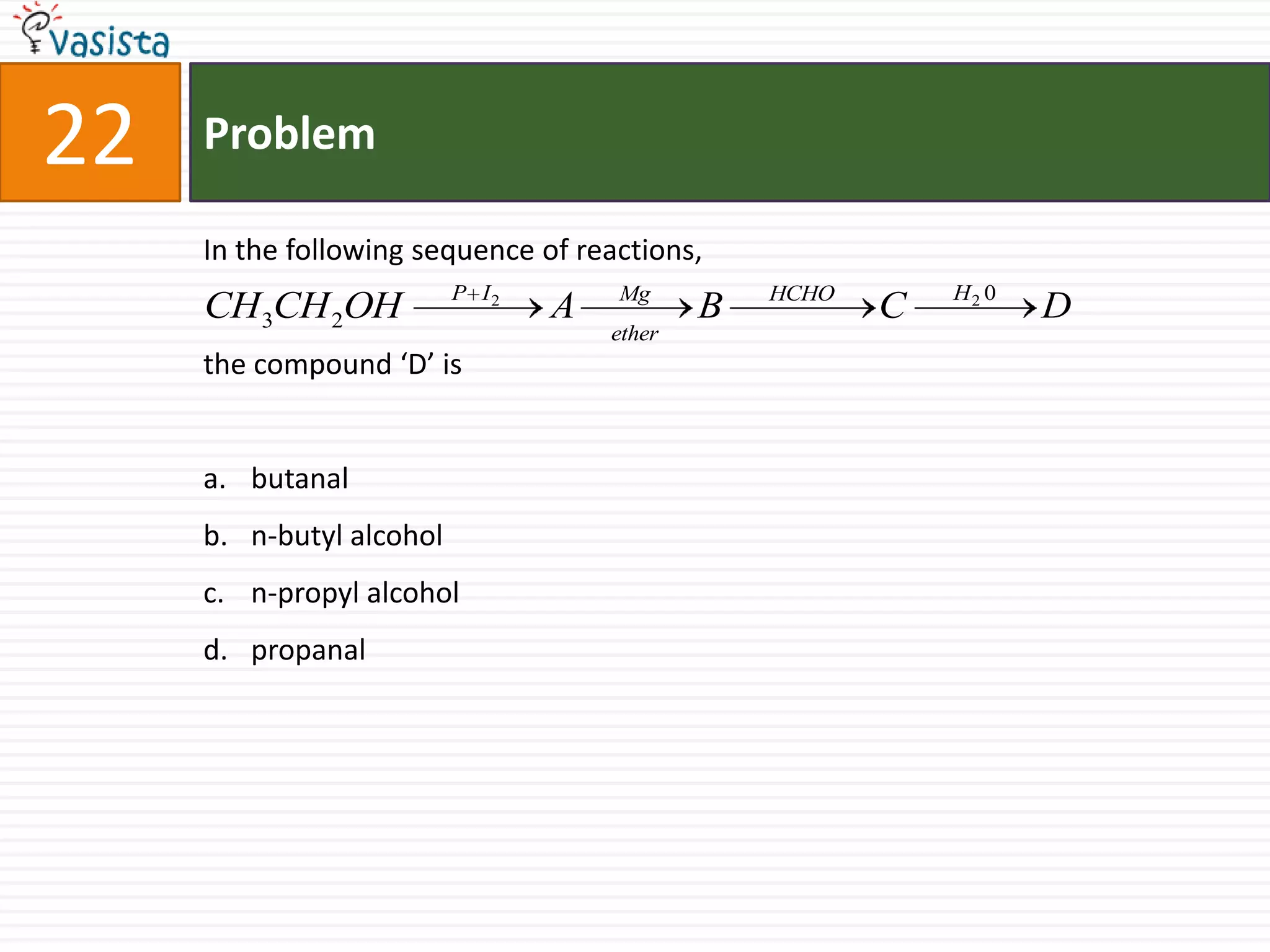
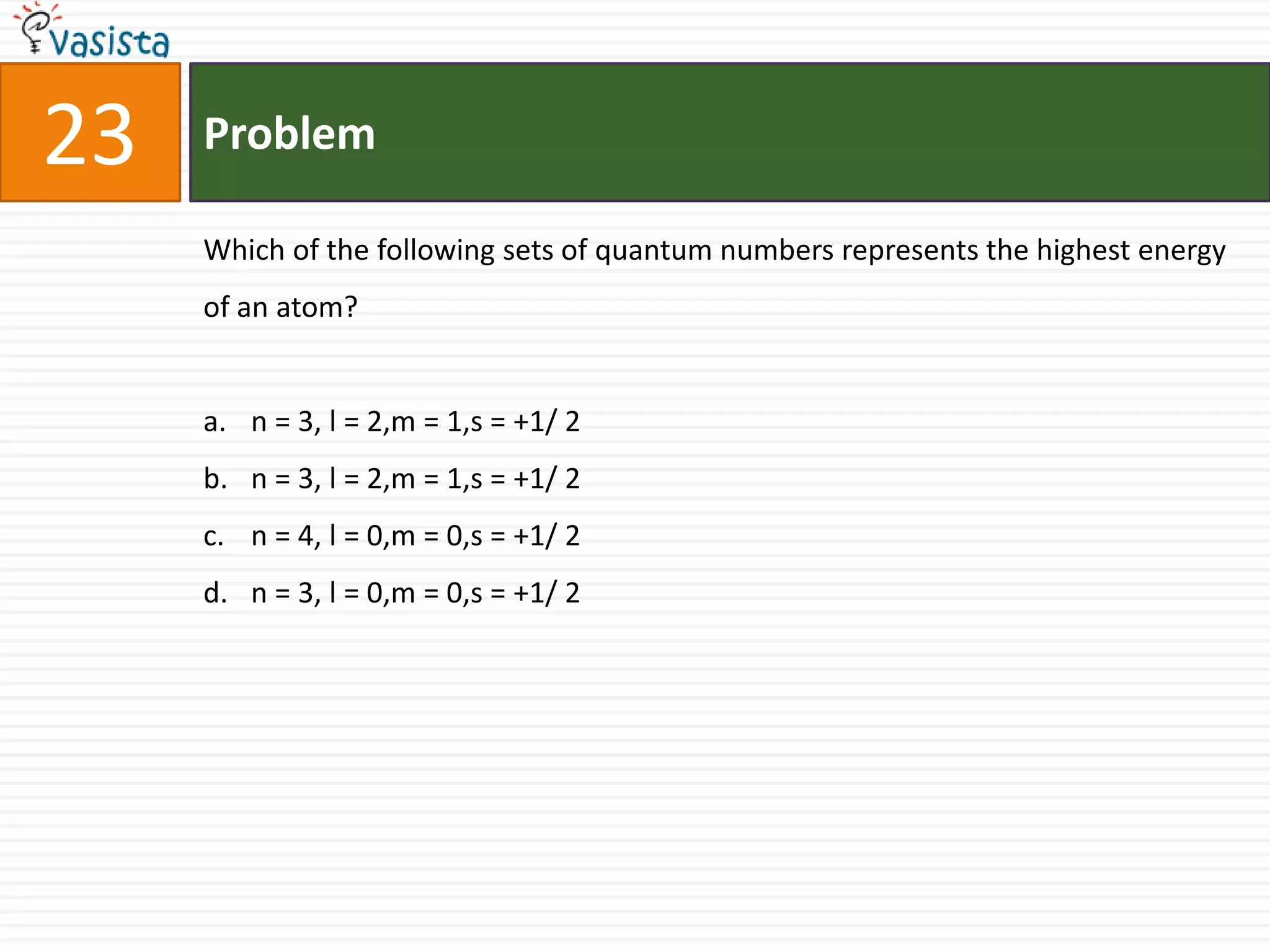
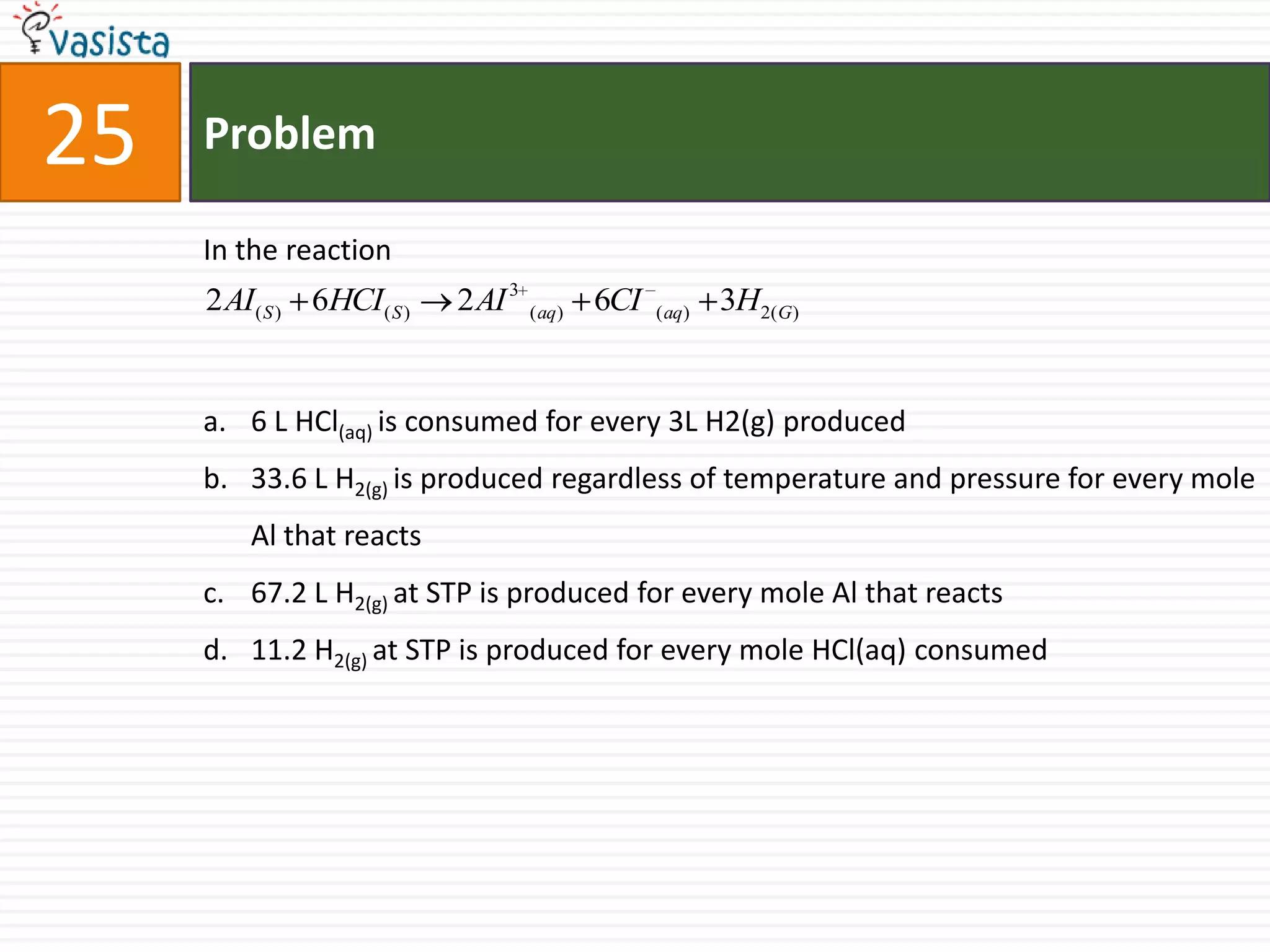
The document is a past paper for the AIEEE chemistry exam from 2007 containing 40 multiple choice questions related to chemistry concepts and calculations. The questions cover topics like thermodynamics, electrochemistry, acid-base chemistry, organic chemistry reactions, and properties of elements.



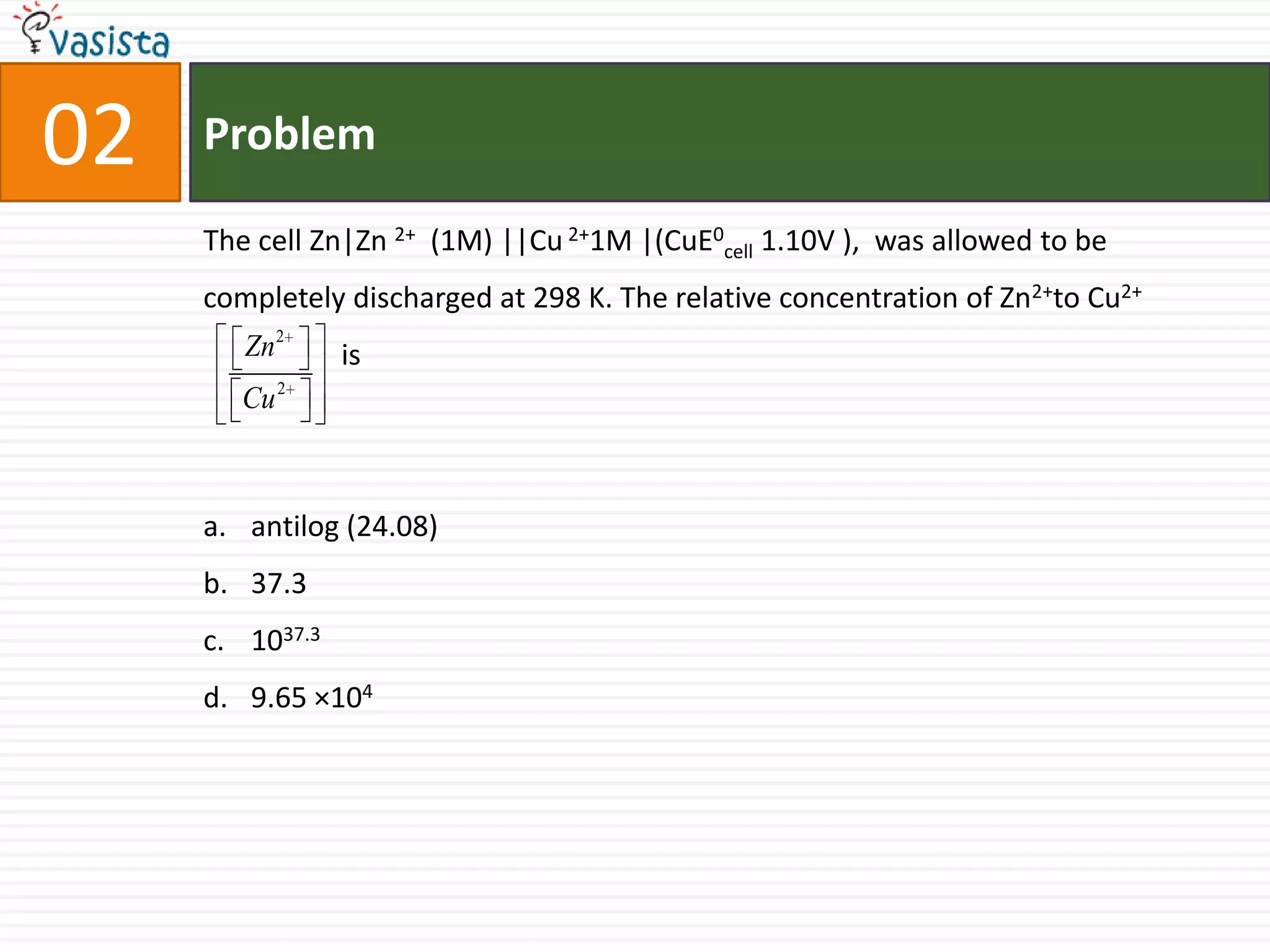



![Problem06Which one of the following has a square planar geometry?[CoCl 4 ]2- [FeCl 4 ] 2-[NiCl 4 ] 2-[PtCl4 ] 2-](https://image.slidesharecdn.com/aieee-chemistry-2007-110928052030-phpapp01/75/Aieee-chemistry-2007-8-2048.jpg)