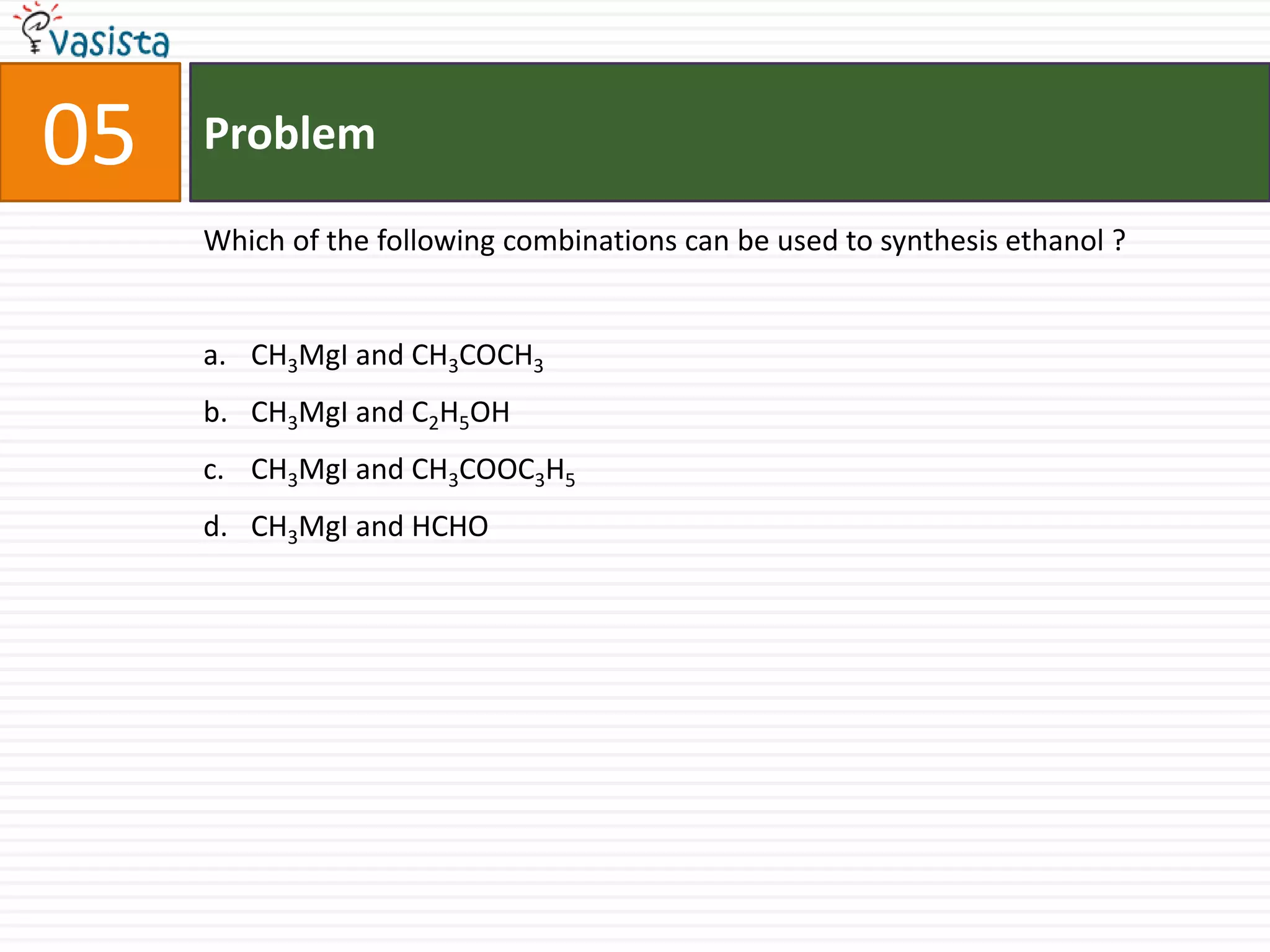
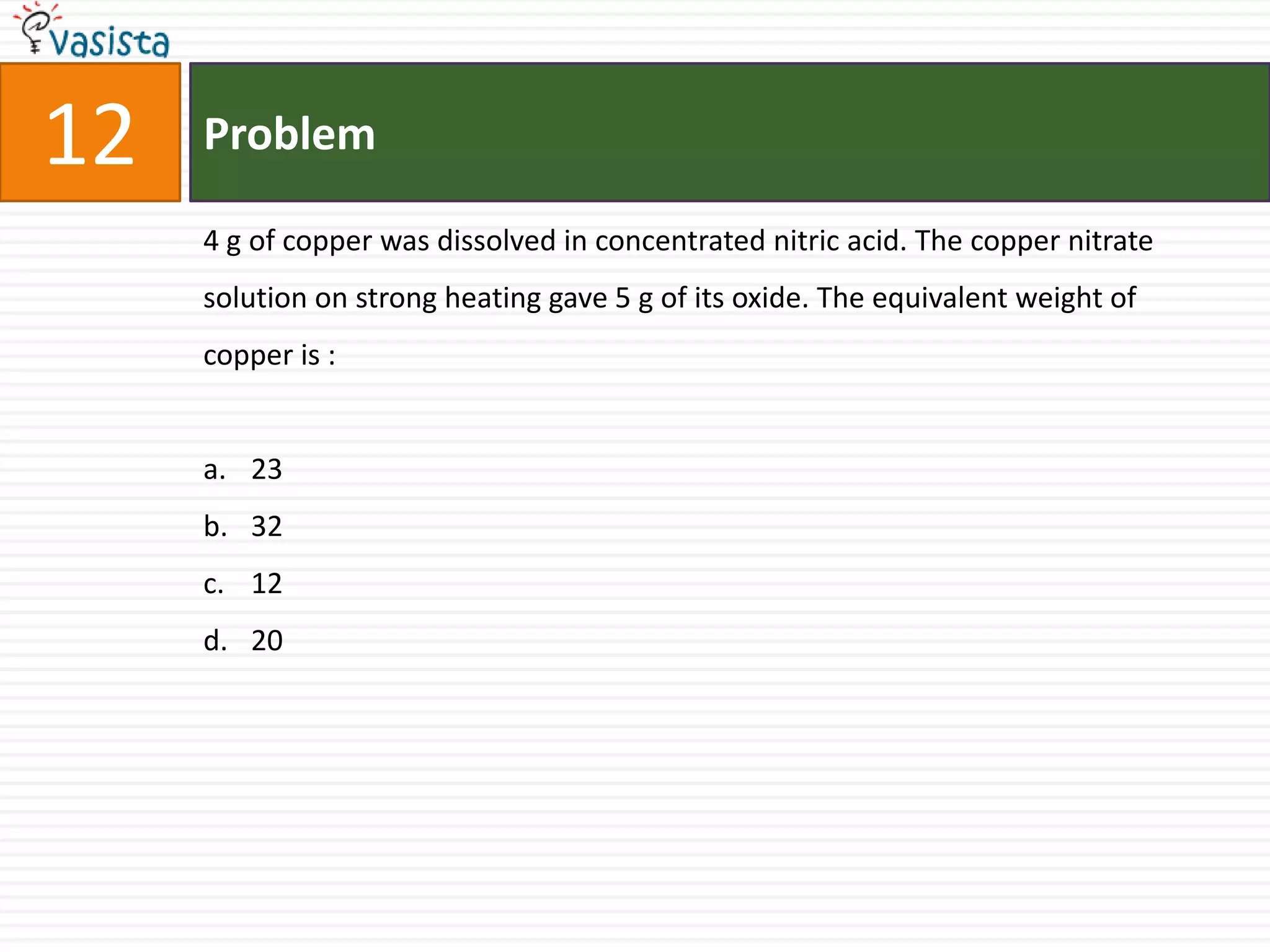
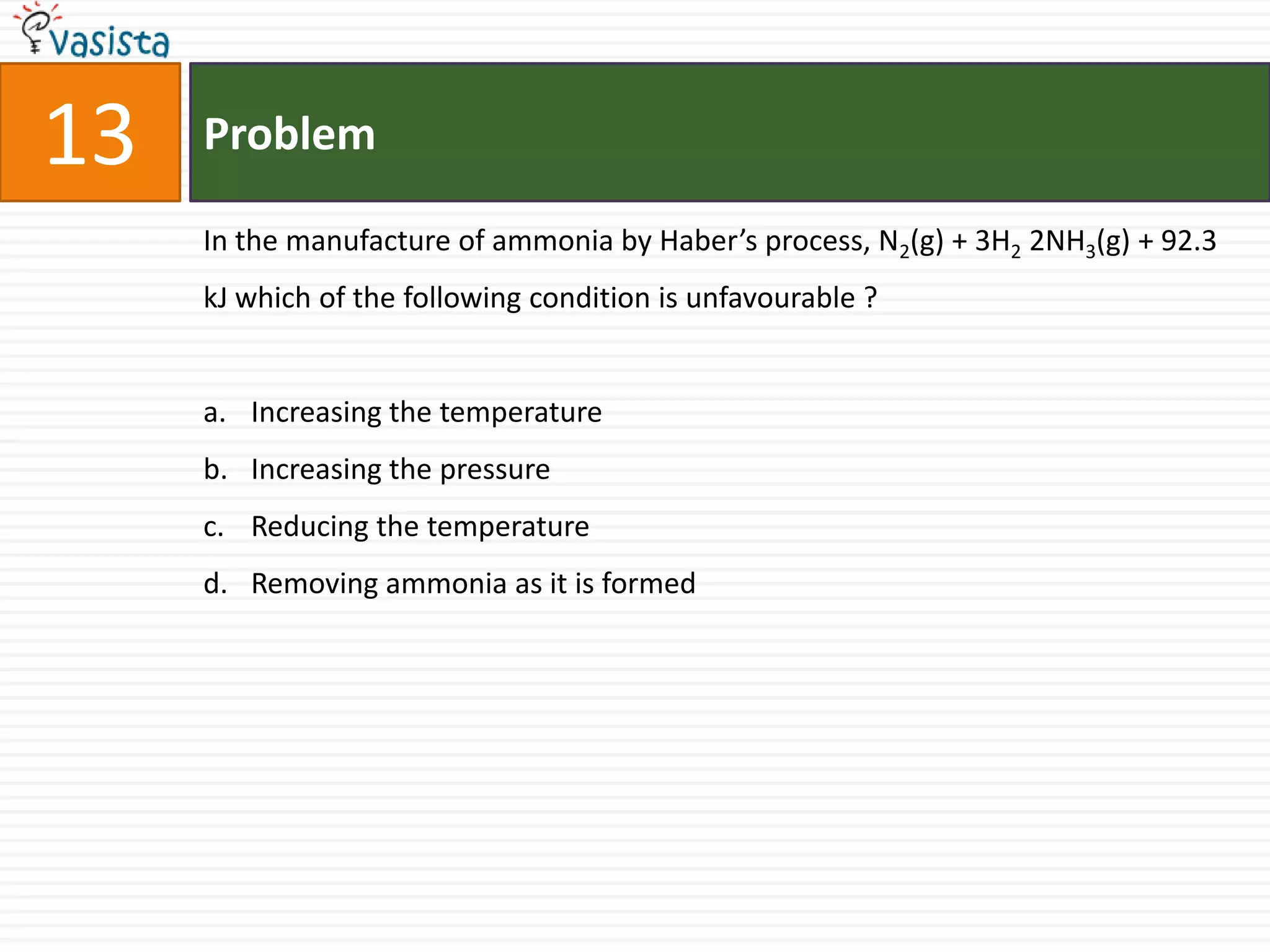
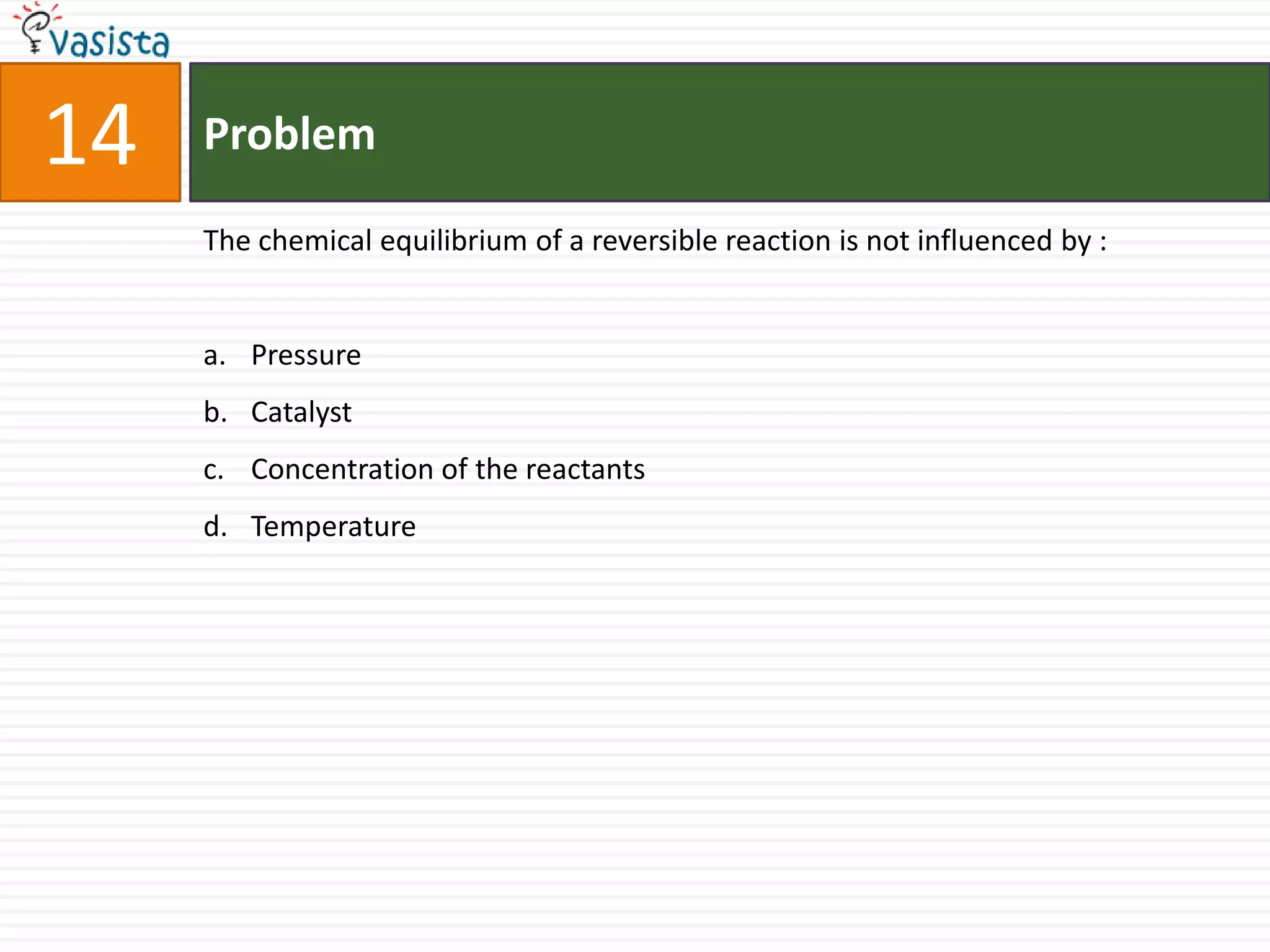
This document contains an unsolved chemistry paper from 2006 containing 40 multiple choice questions. The questions cover topics in chemistry including acids and bases, stoichiometry, organic chemistry, equilibrium, thermodynamics, and more. For each question, four answer choices are provided and the test-taker must select the single correct answer.