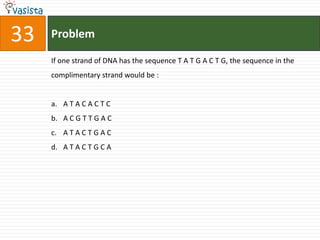
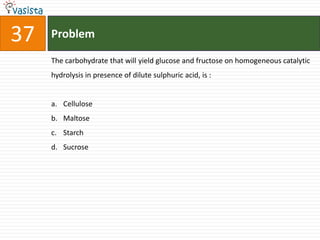
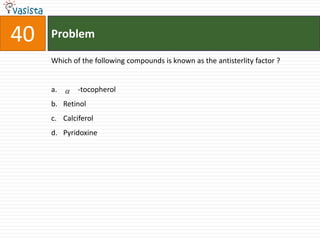
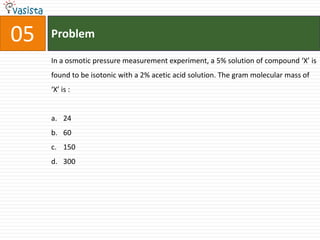
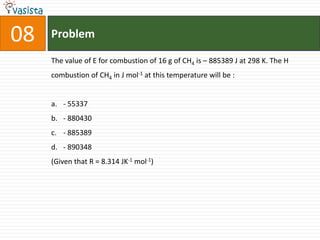
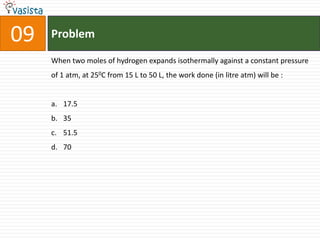
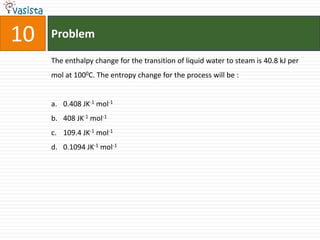
The document provides a chemistry exam paper from 2005 containing 40 multiple choice questions testing knowledge of topics including stoichiometry, atomic structure, thermodynamics, organic chemistry, coordination chemistry, and biochemistry. The questions cover the calculation of molarity from mass and volume, assigning electronic configurations to atoms and ions, determining enthalpy and entropy values in chemical reactions, identifying reaction mechanisms and products, describing properties of coordination complexes and group 14 elements, outlining enzyme functions, and more.




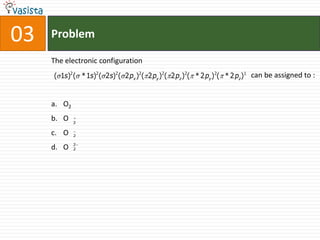



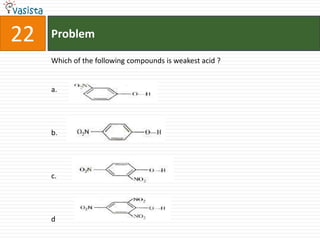
![11 Problem
The entropy change for the reaction H2(g) + Cl2(g) 2 HCl(g), will be :
a. 20 JK-1 mol-1
b. - 20 JK-1 mol-1
c. 167 JK-1 mol-1
d. -167 JK-1 mol-1
[Given that S0 (HCl) = 187 JK-1 mol-1, So(H2) = 131 JK-1 mol-1, and So(Cl2) = 233
JK-1 mol-1]](https://image.slidesharecdn.com/chemistry-2005-120103061026-phpapp02/85/AMU-Chemistry-2005-13-320.jpg)
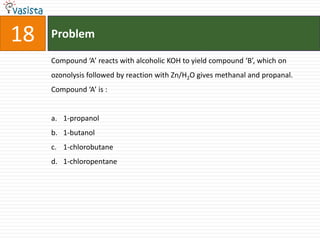
![20 Problem
HBr AgCN [H ]
In the sequence of reactions C2 H4 X Y Z, compound ‘Z’
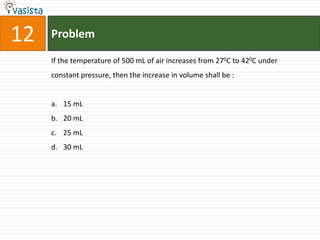
2H / Ni
is :
a. N-methyl ethanamine
b. N-propylamine
c. N,N-dimehtylamine
d. Ethyl cyanide](https://image.slidesharecdn.com/chemistry-2005-120103061026-phpapp02/85/AMU-Chemistry-2005-22-320.jpg)


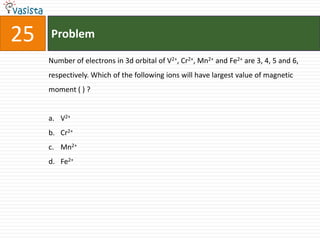
![26 Problem
The co-ordination compounds [Co(NH3)6]3+ [Cr(CN)6]3- and [Co(NH3)6]3+
[Cr(CN)6]3- are the examples of :
a. Linkage isomerism
b. Co-ordination isomerism
c. Ionization isomerism
d. Geometrical isomerism](https://image.slidesharecdn.com/chemistry-2005-120103061026-phpapp02/85/AMU-Chemistry-2005-28-320.jpg)