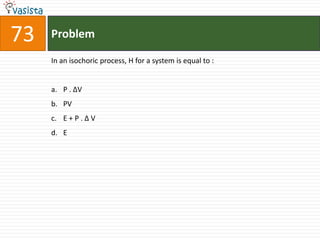
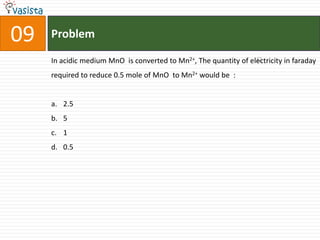
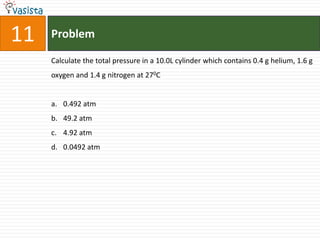
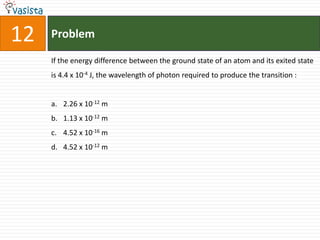
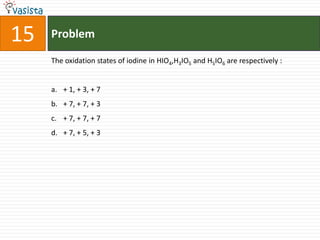
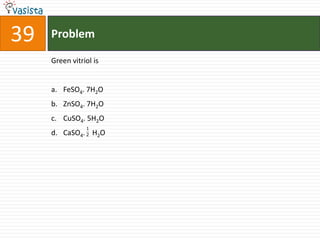
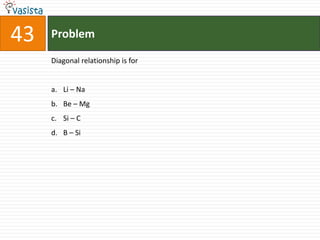
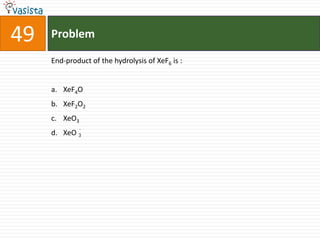
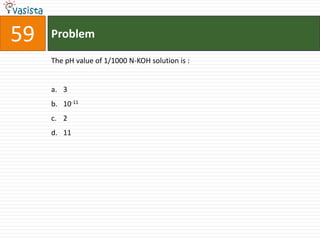
This document contains an unsolved chemistry exam from 2006 consisting of 59 multiple choice questions testing concepts related to general chemistry, organic chemistry, and inorganic chemistry. The questions cover topics such as chemical bonding, stoichiometry, thermodynamics, reaction mechanisms, and properties of common substances and elements.





















































![70 Problem
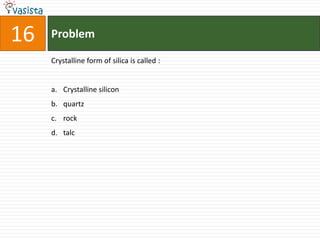
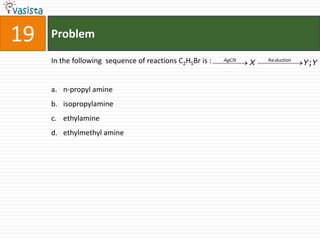
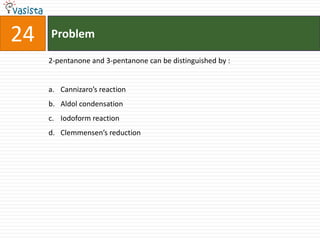
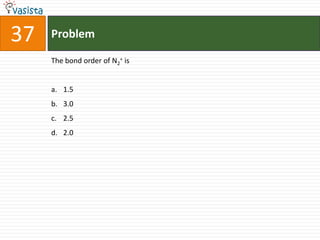
The IUPAC name of K2 [PtCl6] is :
a. Hexachloroplatinate potassium
b. Potassium hexachloroplatinate (IV)
c. Potassium hexachloroplatinate
d. Potassium hexachloroplatinum (IV)](https://image.slidesharecdn.com/2006-111214014710-phpapp02/85/UPSEE-Chemistry-2006-Unsolved-Paper-72-320.jpg)