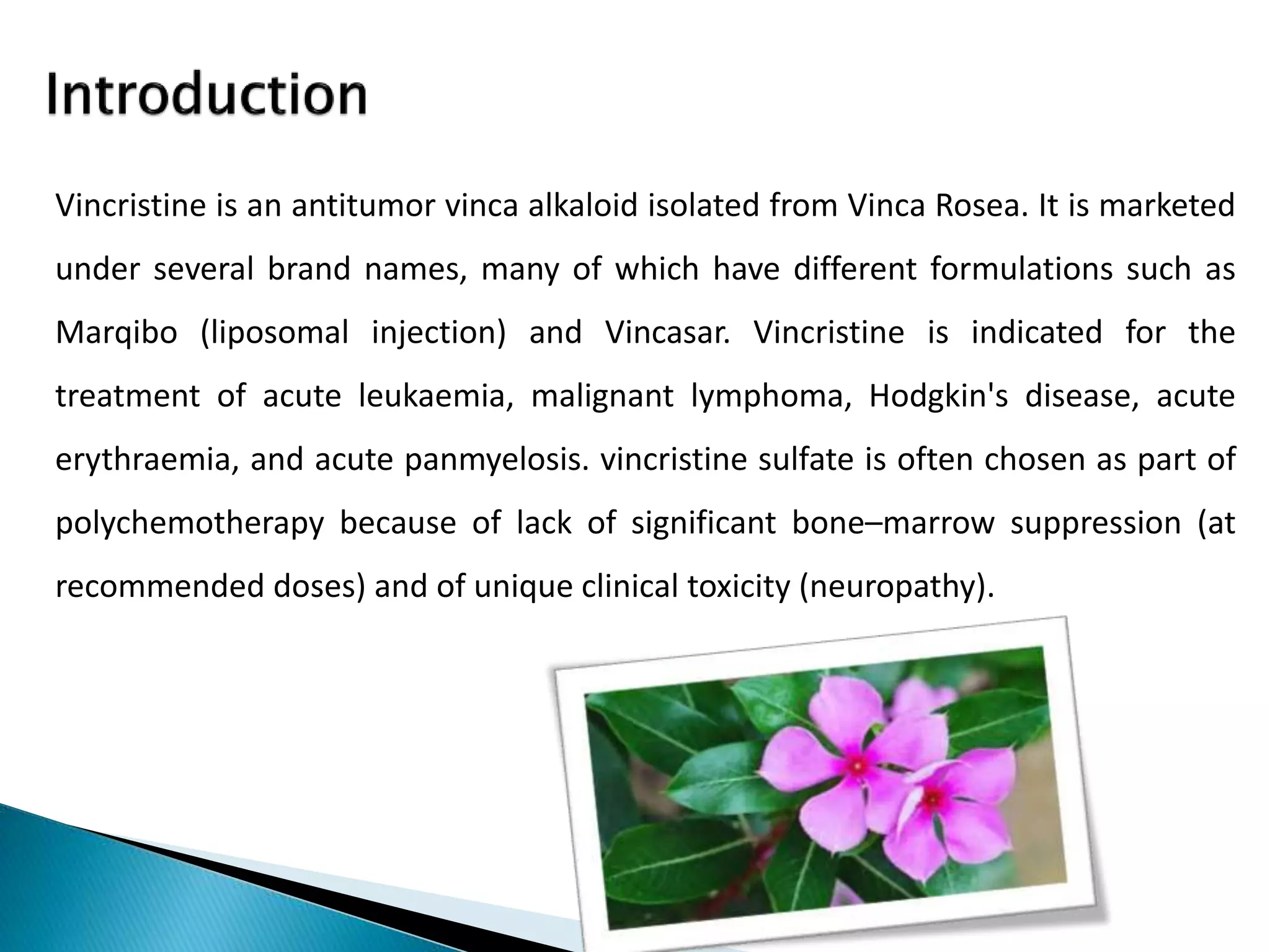
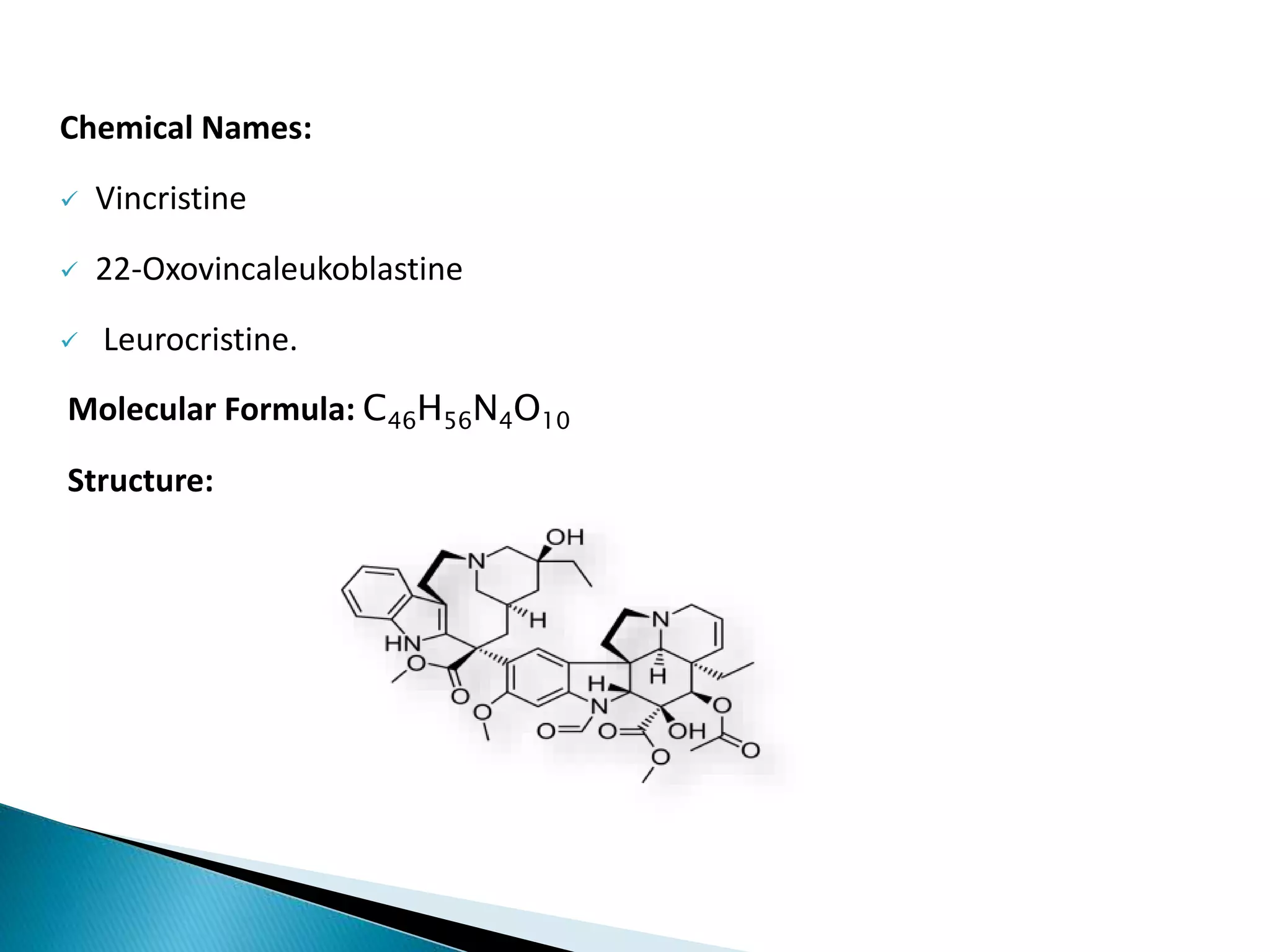
Vincristine is an antitumor alkaloid isolated from Vinca rosea that is used to treat various cancers. It works by inhibiting cell division through interaction with tubulin, which arrests mitosis. Common side effects include neuropathy, infection risk, and hair loss. Vincristine is administered intravenously and distributed widely through the body, where it remains tightly bound, before being metabolized in the liver and excreted primarily in feces.