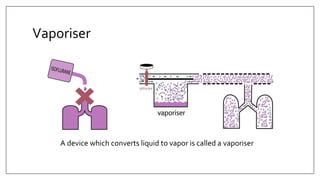
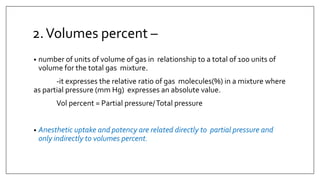
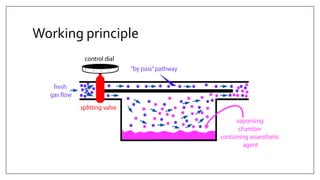
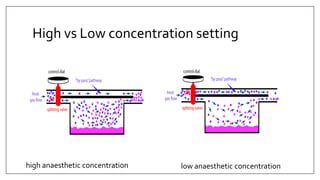
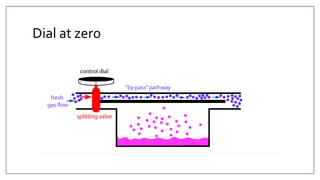
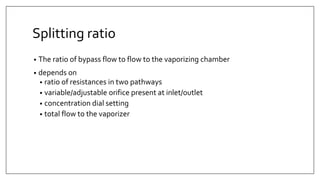
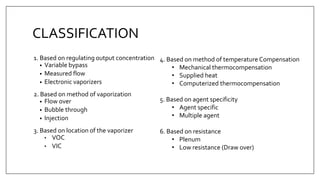
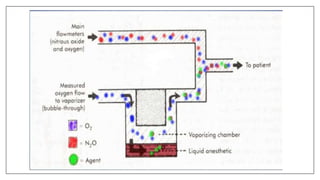
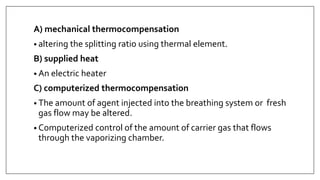
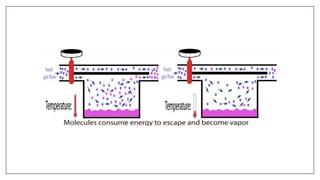
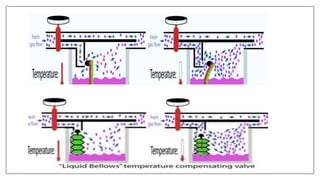
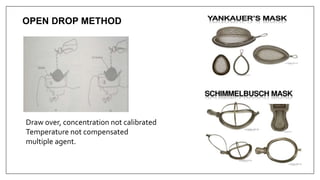
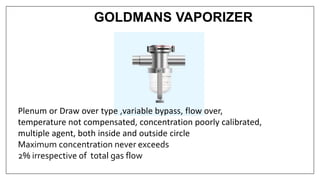
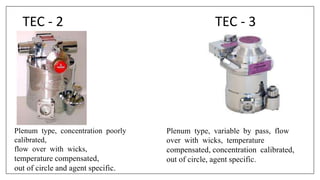
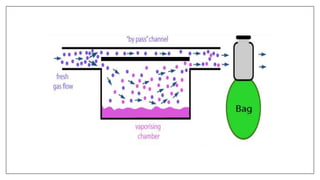
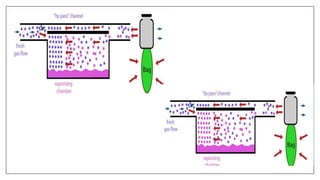
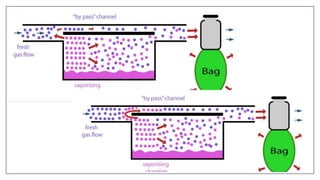
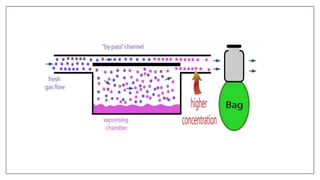
1) Vaporizers are devices that convert liquid anesthetic agents into vapor and add a controlled amount of vapor to the breathing system.
2) There are various types of vaporizers that use different mechanisms for vaporization and temperature compensation. Common vaporizers include the TEC, Goldman, and Copper Kettle vaporizers.
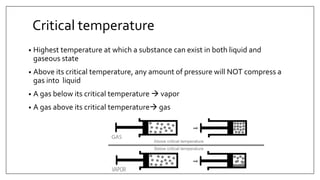
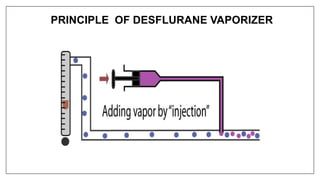
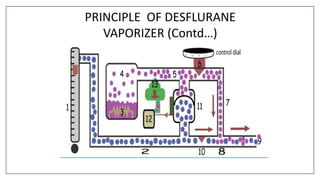
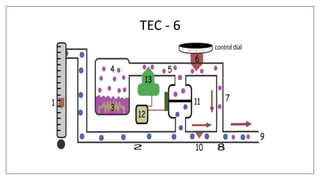
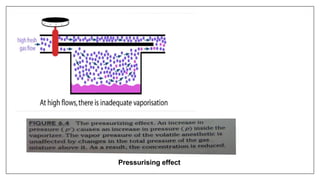
3) Special desflurane vaporizers are required due to desflurane's high vapor pressure, as standard vaporizers could result in dangerously high concentrations being delivered to the patient.