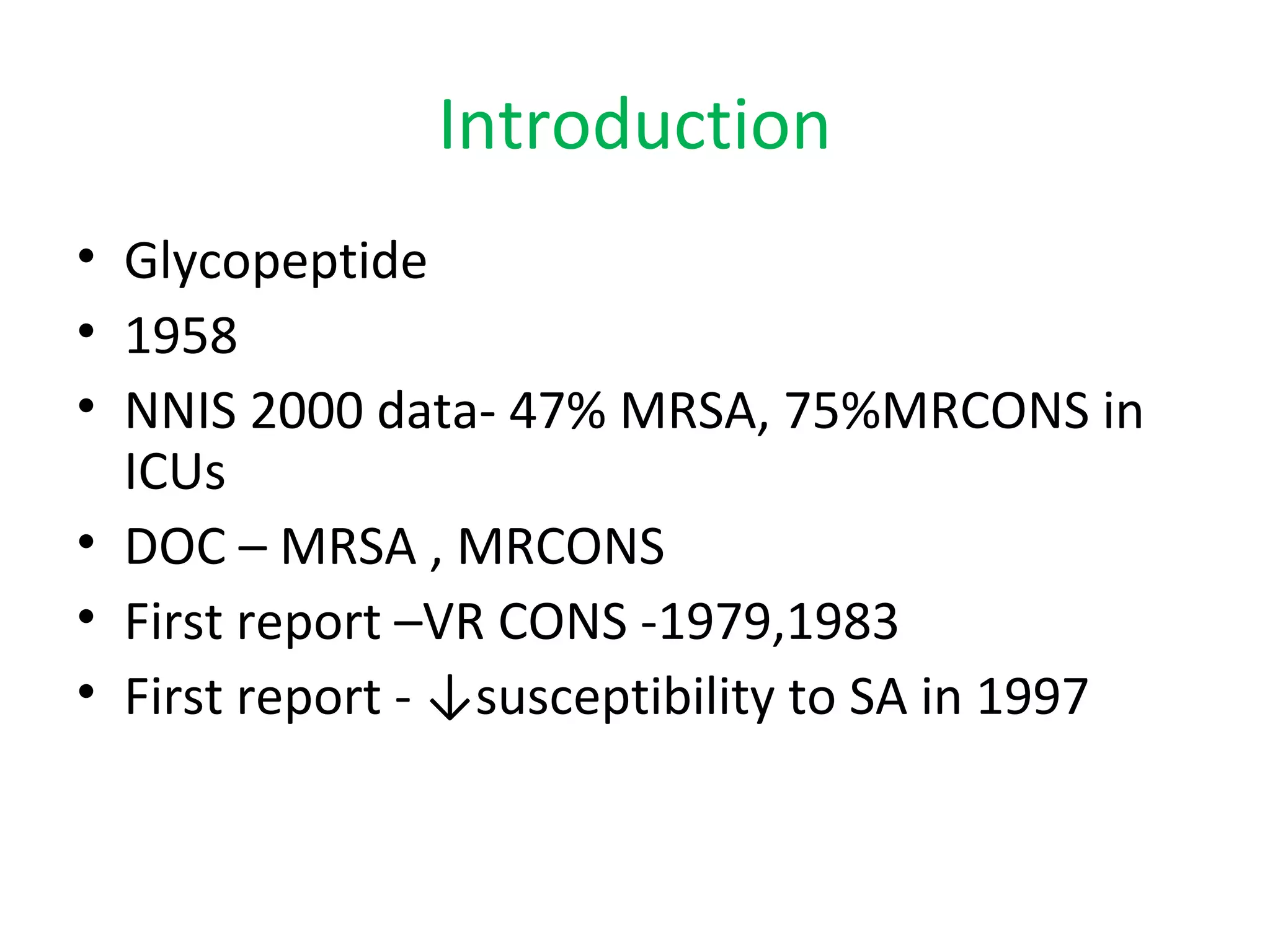
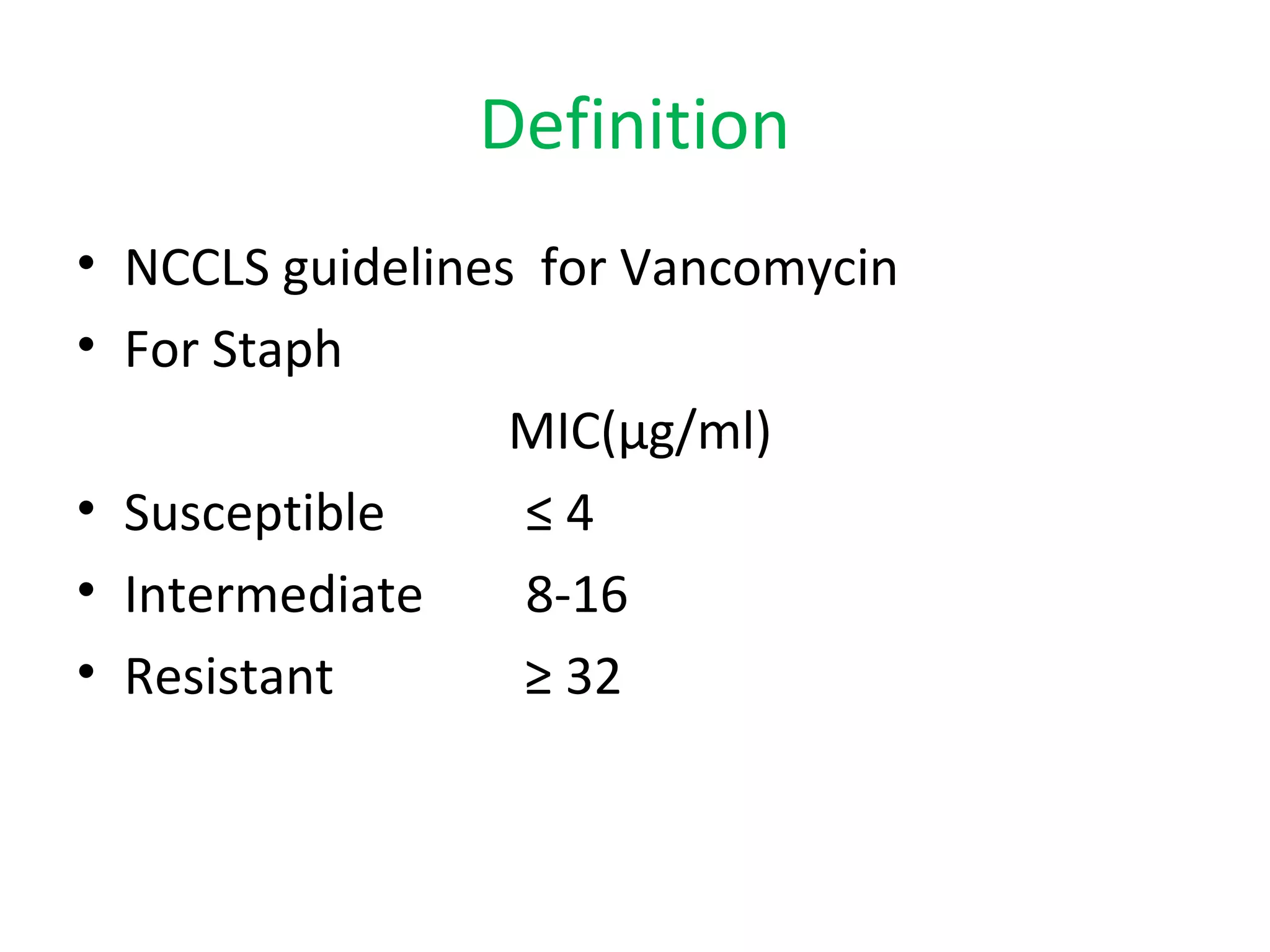
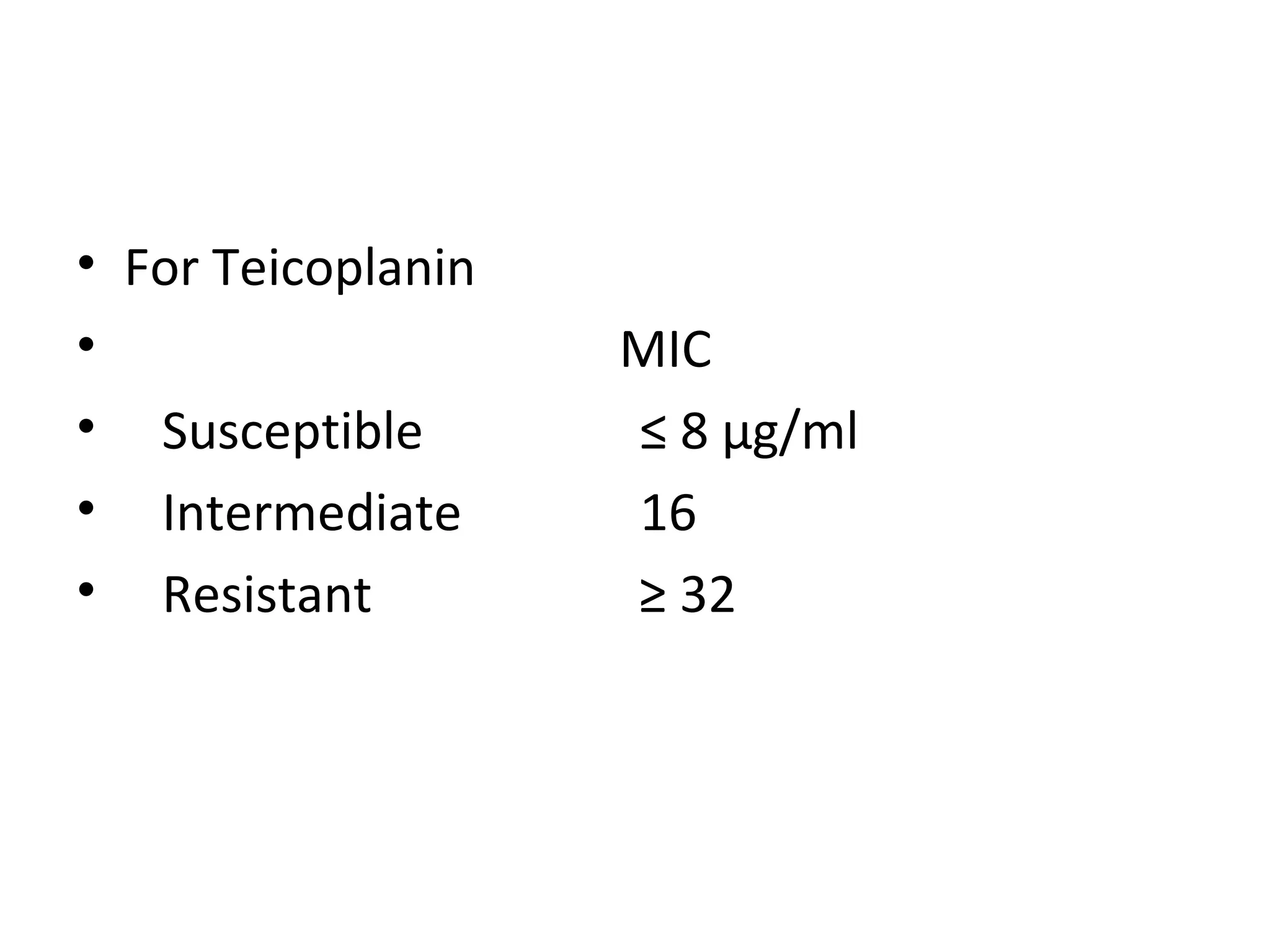
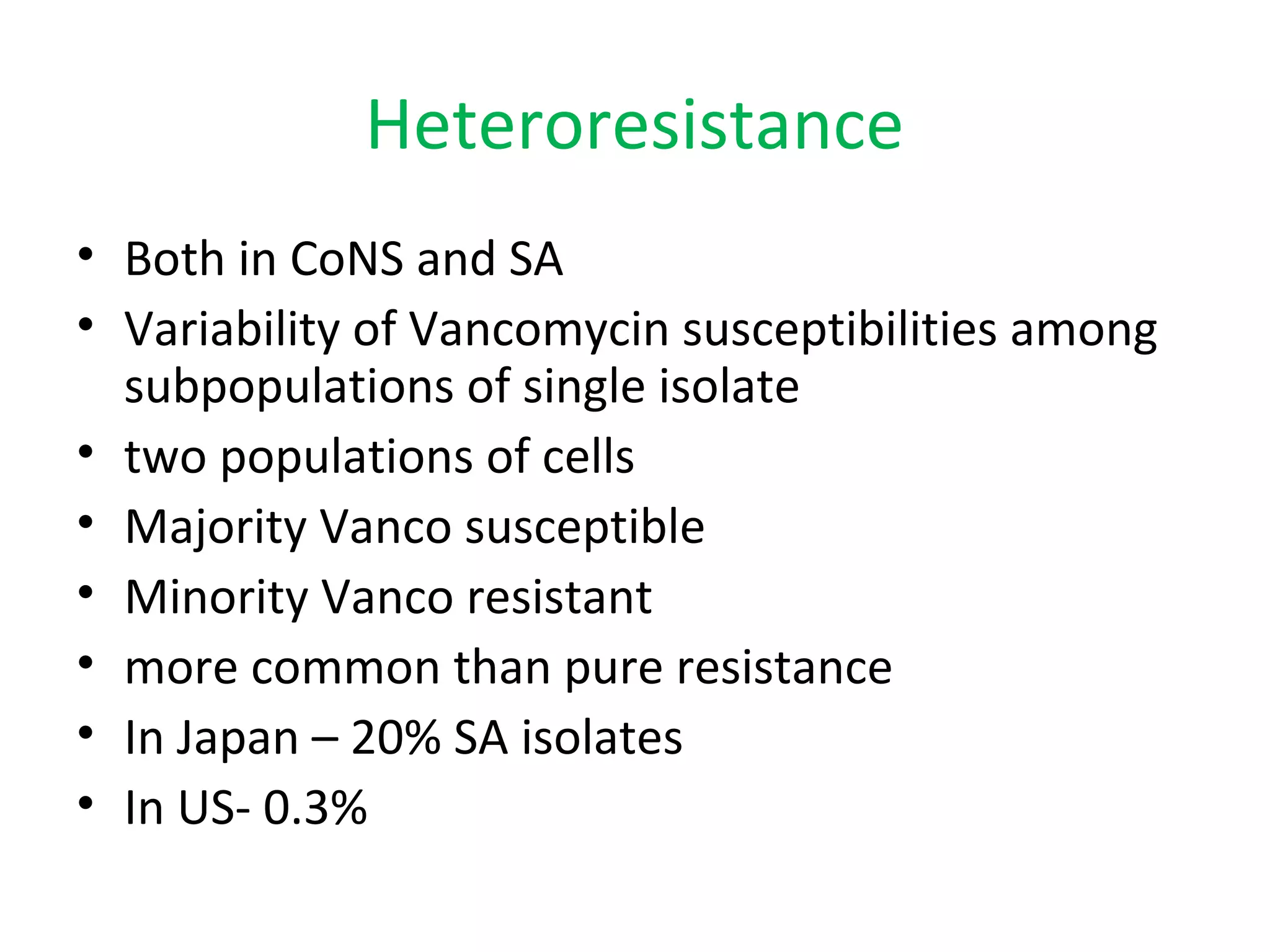
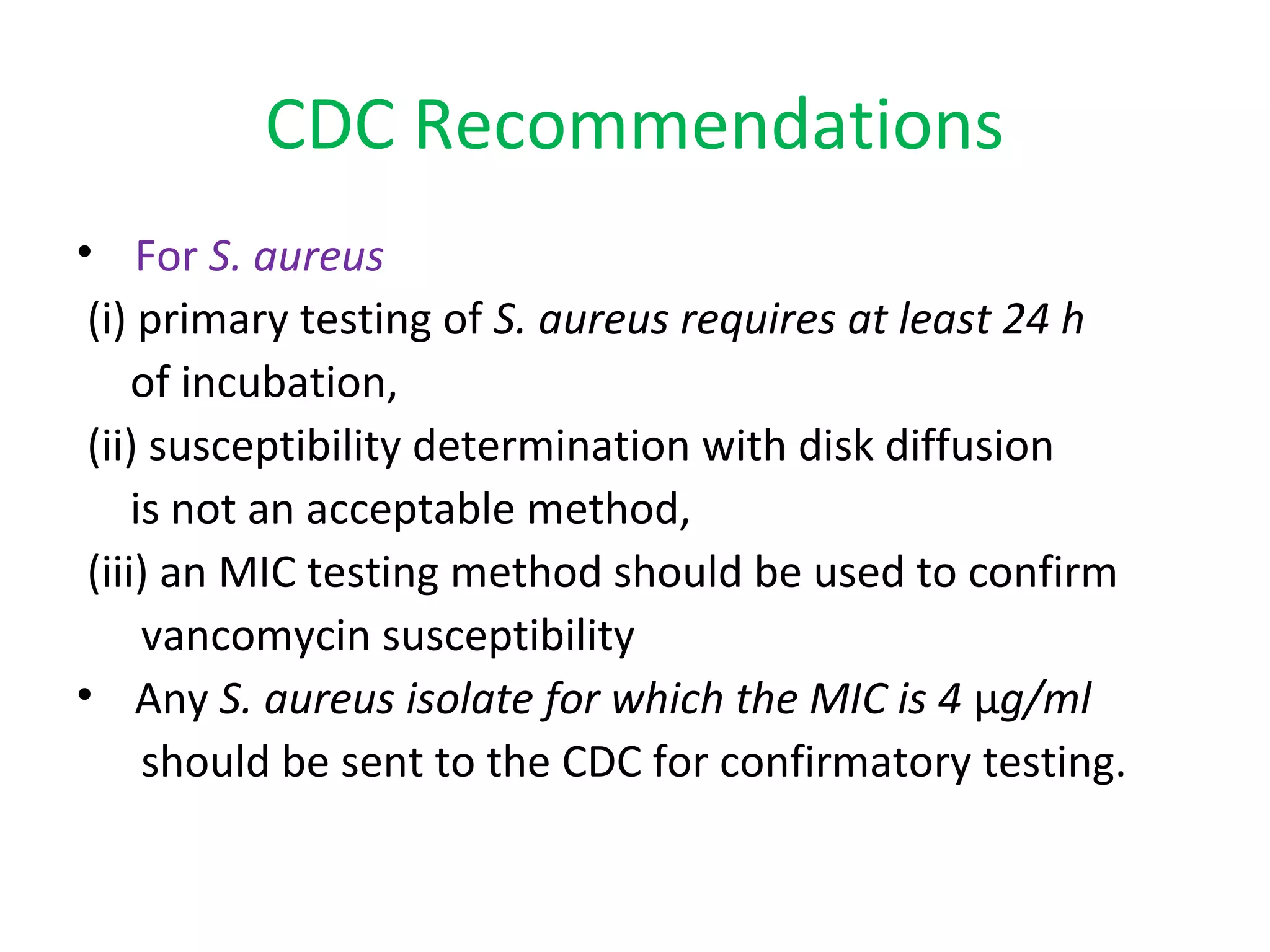
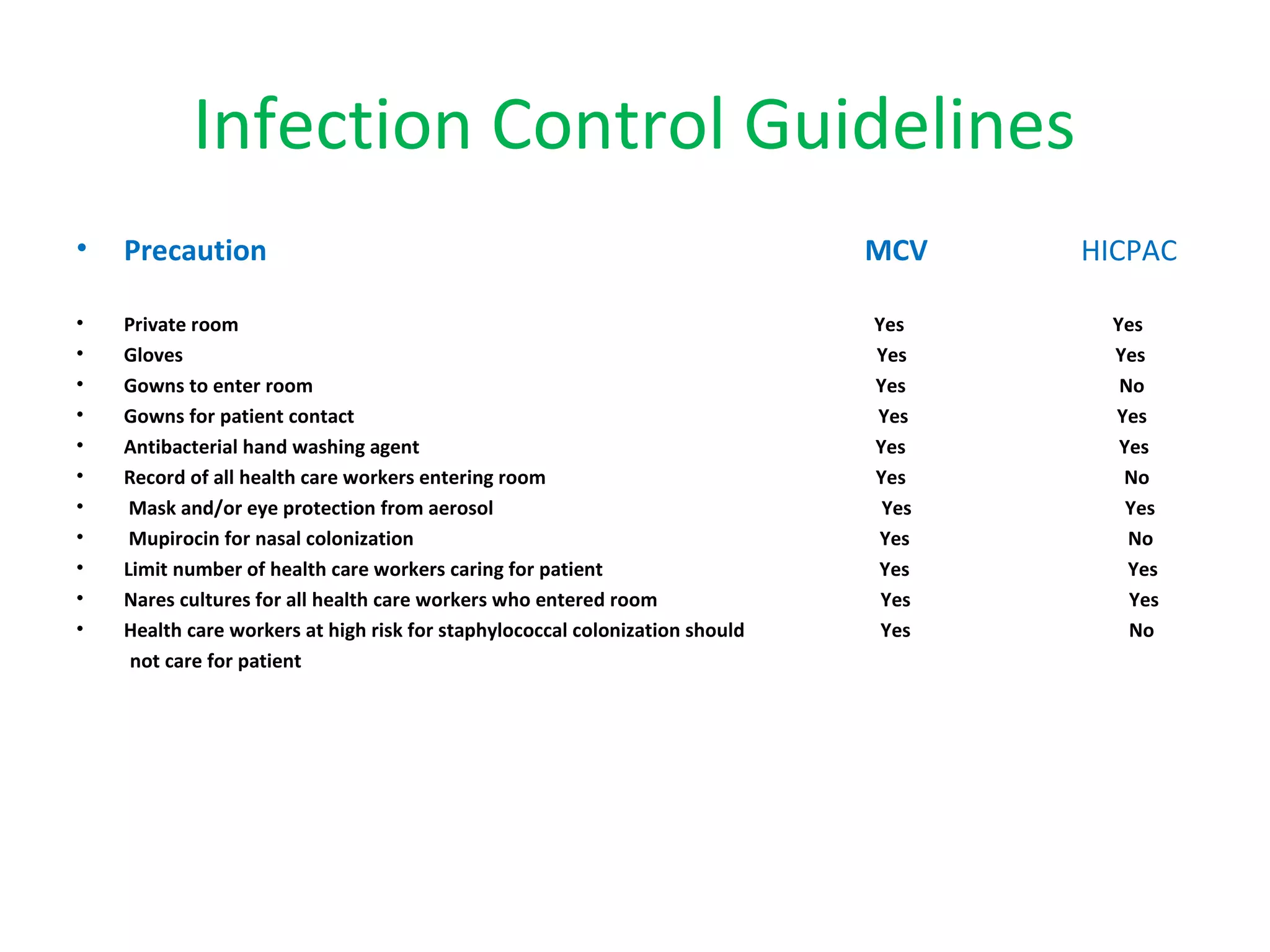
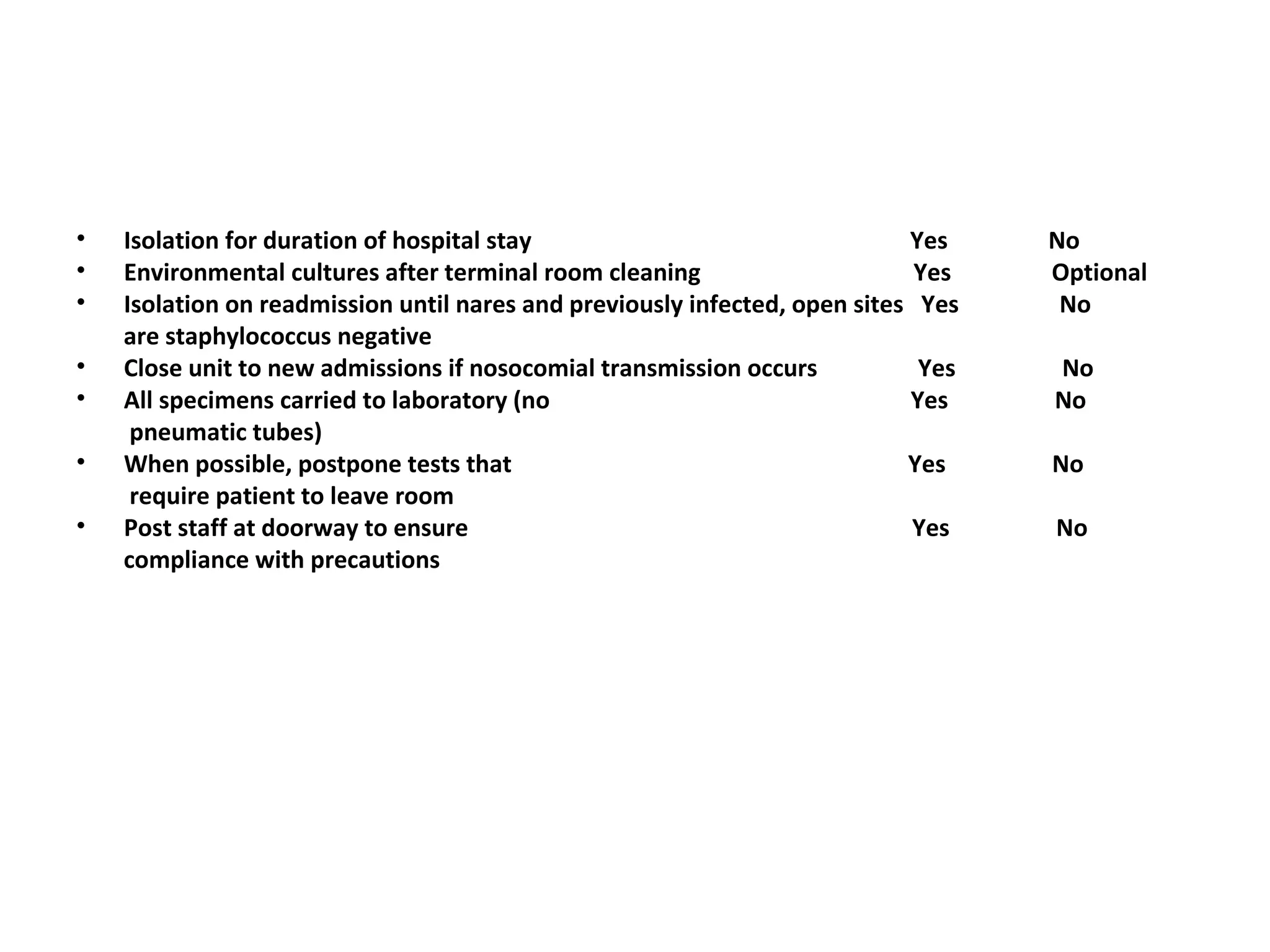
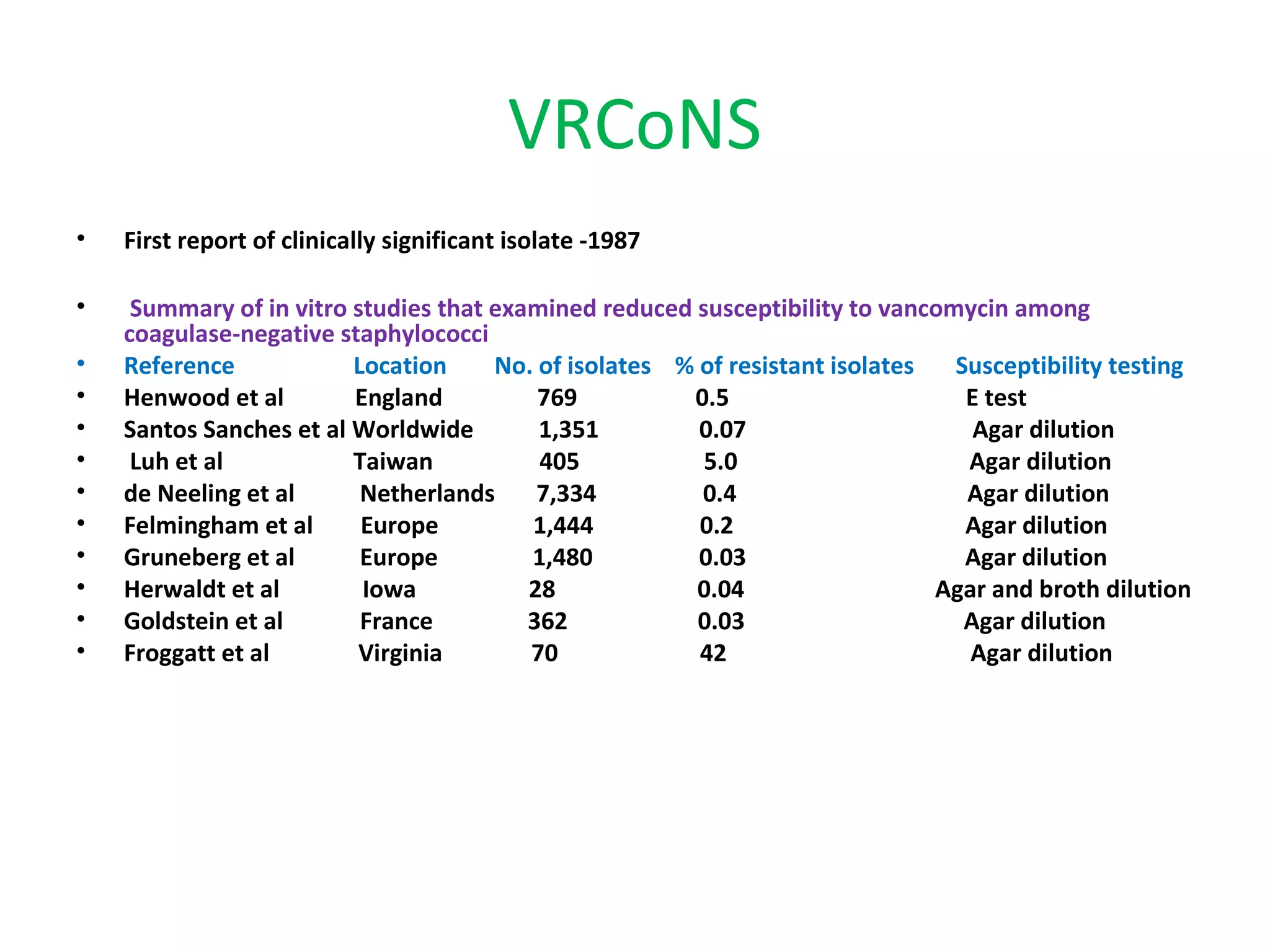
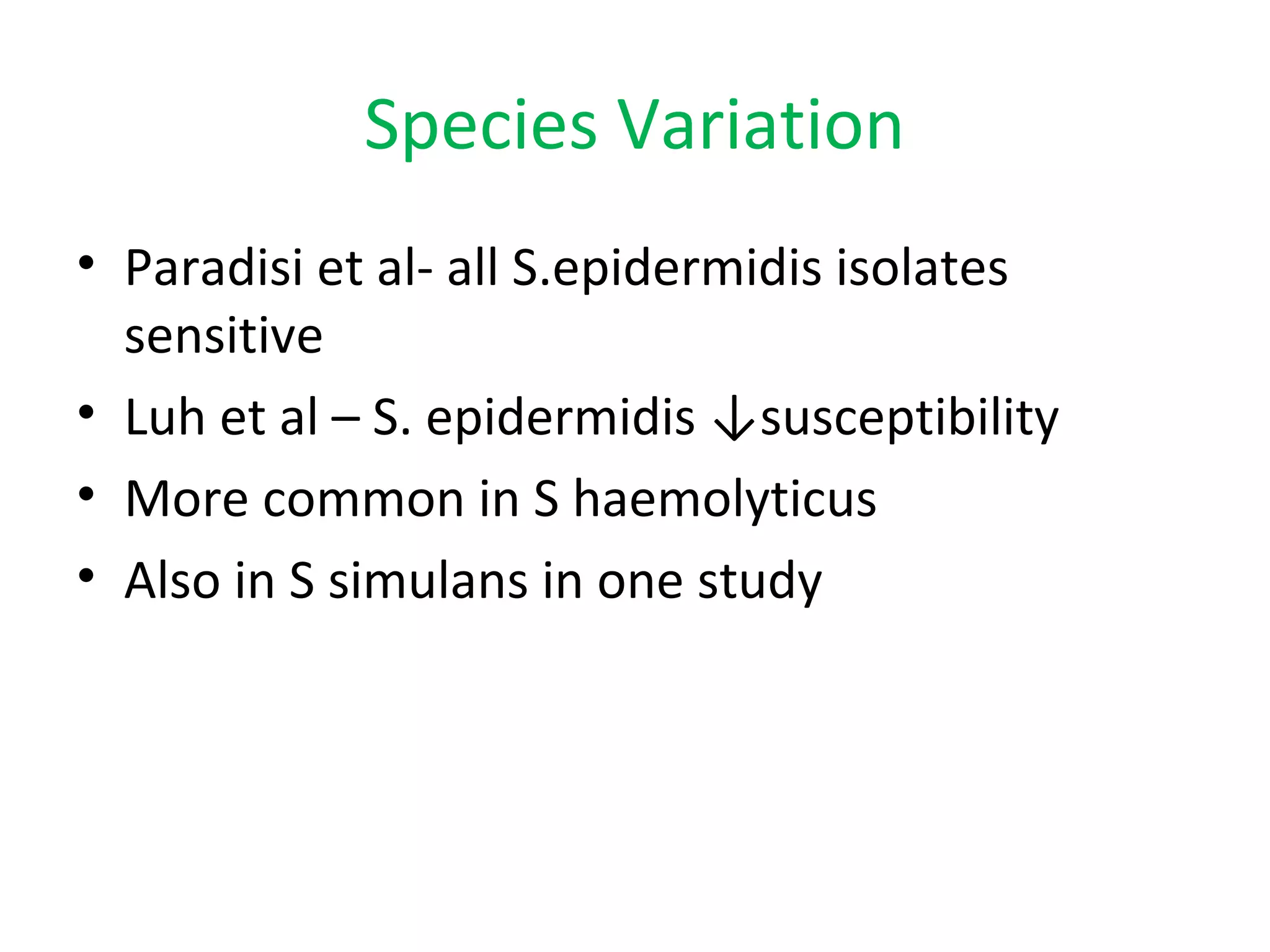
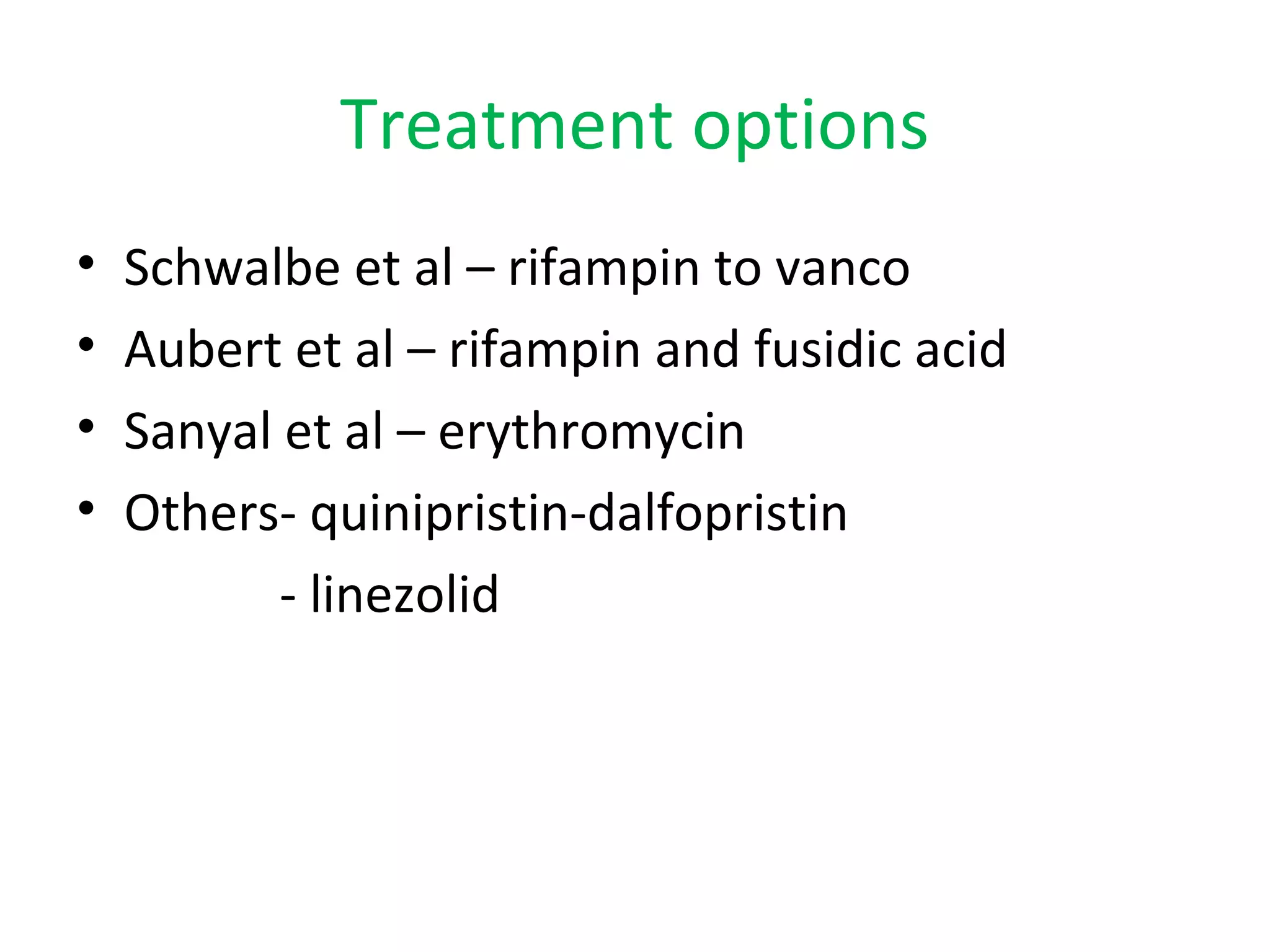
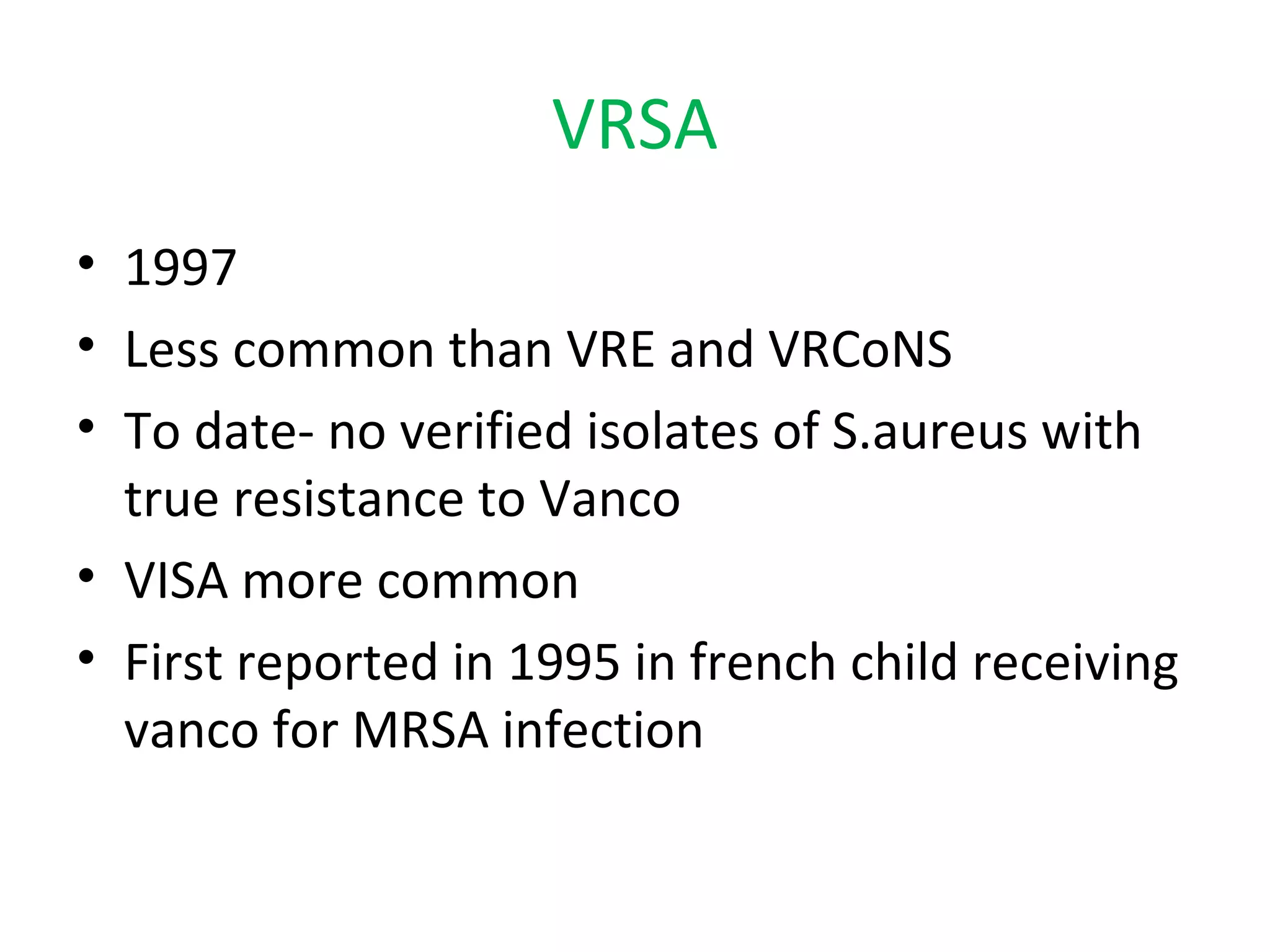
This document summarizes information about vancomycin resistance in staphylococci. It discusses the definition of vancomycin resistance based on MIC levels, mechanisms of resistance such as cell wall alterations, risk factors for resistance like glycopeptide exposure, and recommendations for screening and treating resistant strains. Several studies reporting rates of vancomycin resistance in coagulase-negative staphylococci and rare cases of vancomycin resistance in Staphylococcus aureus are also summarized.