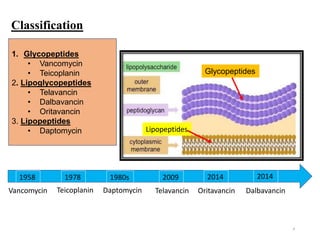
This document discusses glycopeptides, lipoglycopeptides, and lipopeptides used to treat gram-positive bacterial infections. It begins with classifications of these drugs and mechanisms of action. Specifically, it focuses on vancomycin as the first glycopeptide antibiotic discovered from soil samples. It describes vancomycin's structure, mechanism of inhibiting cell wall synthesis, and pharmacokinetics. The document also covers the spectrum of activity and clinical uses of vancomycin and risks of resistance.














![17
Heteroresistance VISA (hVISA):“precursor of VISAs”
Subpopulation of cells which grow at high vancomycin MIC (>2) on vancomycin-containing agar.
• Mutations in 2-component regulatory system that control
several genes involved in cell wall homeostasis:-
1) VraSR system –histidine-kinase (VraS) and response
regulator (VraR) - participate in cell wall turnover and
cell wall envelope stress response.
2) WalkR –decreases autolytic activity in cell walls
When testing isolates at standard inoculum certain
subpopulation of cells are not detected and [“vancomycin
MIC will falsely fall within susceptibility range]”.
Conventional susceptibility tests do not identify
heteroresistance (until much higher fraction of
resistant cells is present)!!!
Population analysis profile (PAP) (determination of number of surviving cells at
increasing antibiotic concentrations) is required for detection.
disk diffusion method only detects strains with very high vancomycin MICs and fails to
detect hVISA isolate.
“MIC determinations by agar or broth dilution or by Etests are recommended for
vancomycin susceptibility.”
Heteroresistance
VISA (hVISA):](https://image.slidesharecdn.com/finalglycopeptidesandlipopeptidessaturday20sep11-211002092232/85/glycopeptides-and-lipopeptides-17-320.jpg)
























