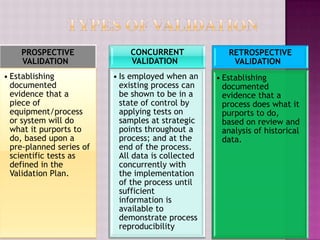
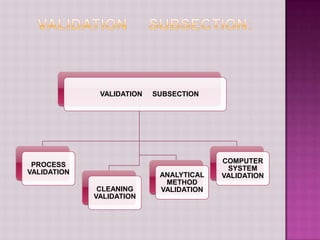
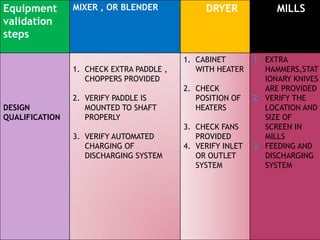
The document discusses validation in the pharmaceutical industry. It defines validation according to regulatory bodies as establishing evidence that a process will consistently produce a product meeting its quality standards. Validation master plans outline the principles and programs for qualifying facilities, processes, analytical methods, and computer systems. The main types of validation are prospective, concurrent, and retrospective. Key aspects of validation covered include process validation, cleaning validation, and analytical method validation. Validation aims to demonstrate processes and methods are fit for their intended purposes.