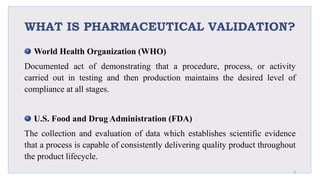
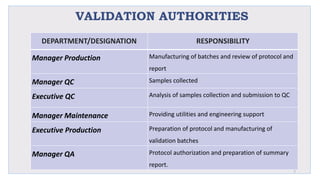
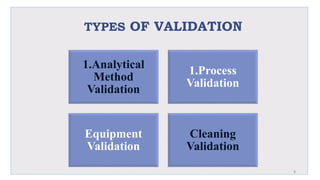
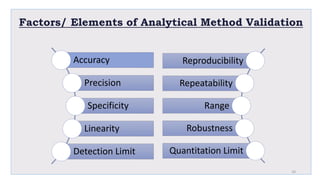
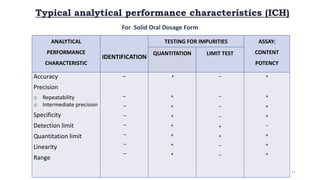
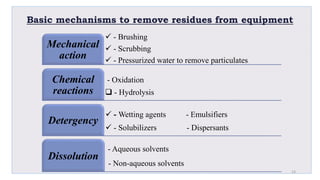
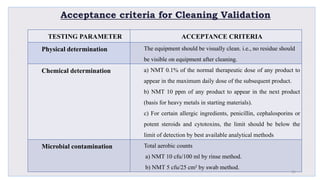
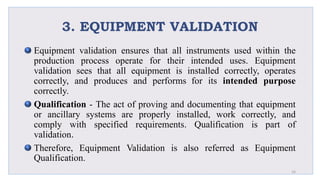
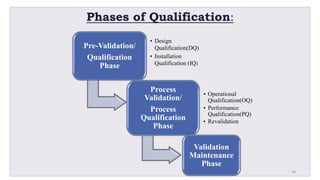
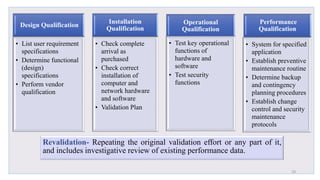
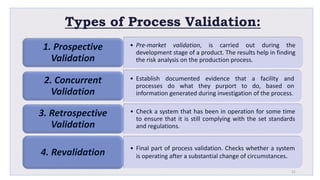
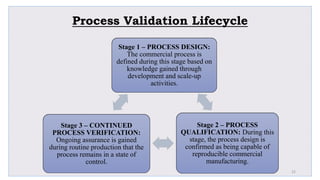
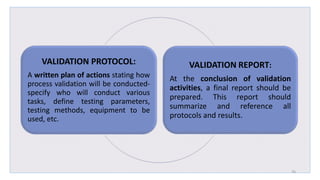
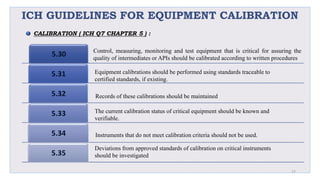
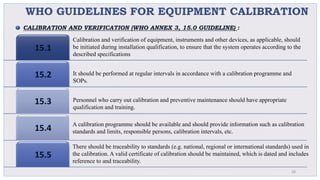
Le document traite de la validation pharmaceutique, définissant son importance pour garantir la qualité des produits tout au long du processus de production, et souligne l'historique de ce concept en réponse à des défaillances dans la stérilité des produits. Il aborde également les autorités responsables, les différents types de validation (y compris l'analyse, le nettoyage et l'équipement) et la nécessité d'un Plan Maître de Validation pour organiser ces processus. Finalement, il mentionne les directives de l'ICH et de l'OMS sur l'étalonnage des équipements nécessaires pour assurer la qualité des produits pharmaceutiques.















![7. Jain K. Process validation of tablet dosage form: A comprehensive review- 2018. The Pharma Innovation Journal
2018; 7(3): 433-438.
8. Sabne A, Sontakke M, Rathi V, Gholve S. Pharmaceutical validation: A Review. JETIR (www.jetir.org); 2023.
9. P. Nethercote. Method validation in pharmaceutical analysis: A Guide to Best Practice, Second, Completely
Revised and Updated Edition. ResearchGate, 2014.
10. Maurya S, Goyal D, Verma C; Cleaning Validation in Pharmaceutical Industry- An Overview; PharmaTutor; 2016;
4(9); 14-20
11. Khan, F. Cleaning validation in pharmaceutical industries – IJRPC 2020, 10(2), 205-214
12. Jindal D, Kaur H, Patil RK, Patil HC. Validation – In pharmaceutical industry: Equipment validation: A brief
review. Adesh University Journal of Medical Sciences & Research. 2020 Dec 19;2(2):94–8.
13. Sumeet S, Gurpreet S. Process validation in pharmaceutical industry: An overview. Journal of Drug Delivery &
Therapeutics [Internet]. 2013(3):184.
31](https://image.slidesharecdn.com/seminarpharmaceuticalvalidation-240116142810-1a12868c/85/Pharmaceutical-Validation-Role-in-Phamaceutical-Industry-31-320.jpg)
