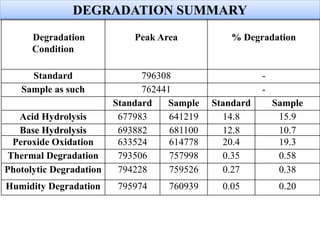
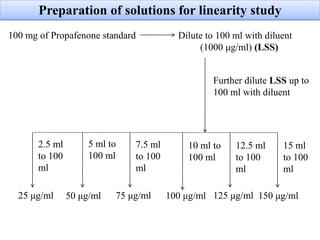
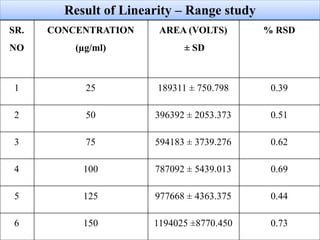
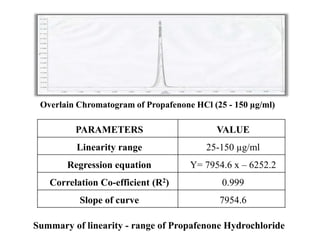
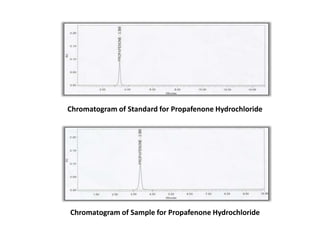
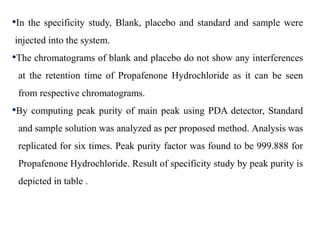
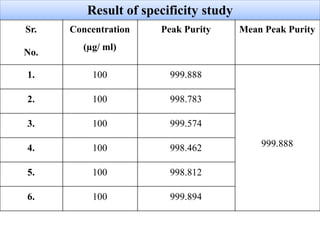
Here are the key IR frequencies identified in the sample that match the reference standard of propafenone:
- C-C stretch at 1186 cm-1
- C=C stretch at 1651 cm-1
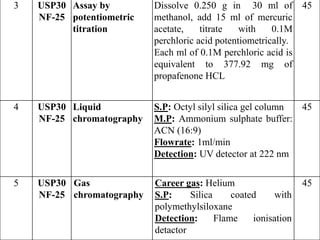
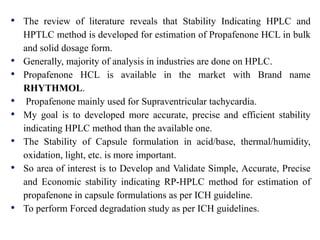
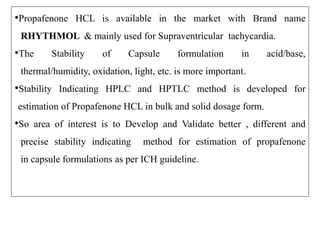
- C-H stretch (symmetric) at 2939 cm-1
- C-H bend at 1328 cm-1
- CH2 stretch (symmetric) at 1369 cm-1
- CH2 bend at 1485 cm-1
- CH3 bend at 1398 cm-1
- C-O stretch at 1100 cm-1
- C=O stretch at 1695 cm-1
- N-H stretch at 3417 cm-1
The IR spectrum

![INTRODUCTION[1-39]](https://image.slidesharecdn.com/da2bc780-6064-4e4b-b4da-7b38a944a722-160726134604/85/VAIBHAV-presentation-final-2-320.jpg)





![Stability Indicating Assay Method (SIAM)
ICH harmonized Tripartite Guideline described Stability Testing of New
Drug Substances and Products [ICH Q1AR2].
According to ICH Q1AR2, SIAM defined as “Method which is reliable,
meaningful and specific means the content of active ingredients, degradation
products and other contents of interest in a drug product can be accurately
measured without interference.”
According to US-FDA stability guideline of 1998, SIAM defined as,
“Validated quantitative analytical methods that can detect the changes with
time in the chemical, physical, or microbiological properties of the drug
substance and drug product, and that are specific so that the contents of
active ingredient, degradation products, and other components of interest
can be accurately measured without interference”](https://image.slidesharecdn.com/da2bc780-6064-4e4b-b4da-7b38a944a722-160726134604/85/VAIBHAV-presentation-final-9-320.jpg)


![DRUG PROFILE[40-43]](https://image.slidesharecdn.com/da2bc780-6064-4e4b-b4da-7b38a944a722-160726134604/85/VAIBHAV-presentation-final-12-320.jpg)
![Name of drug Propafenone
Structure
Mol. formula C21H27NO3.HCL
Mol. Wt. 377.92
CAS No. 54063-53-5
IUPAC name 1-{2-[2-hydroxy-3(propylamino)-propoxy]phenyl}-3-
phenylpropan-1-one
Description Propafenone hydrochloride is a colourless crystals to white
fine crystalline powder with a bitter taste.
Solubility It is slightly soluble in alcohol and chloroform. It is very
slightly soluble in acetone. It is soluble in methanol and hot
water. It is insoluble in diethyl ether and toluene.](https://image.slidesharecdn.com/da2bc780-6064-4e4b-b4da-7b38a944a722-160726134604/85/VAIBHAV-presentation-final-13-320.jpg)


![LITERATURE REVIEW[44-54]](https://image.slidesharecdn.com/da2bc780-6064-4e4b-b4da-7b38a944a722-160726134604/85/VAIBHAV-presentation-final-16-320.jpg)