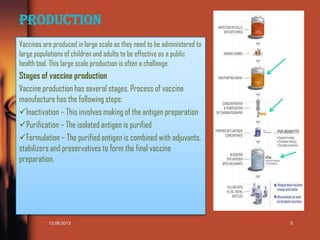
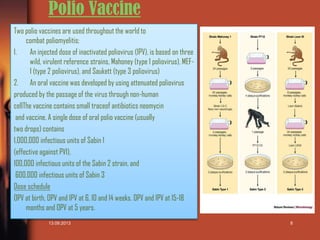
This document is a presentation on vaccines that was created by Sana Shaikh for a class. It includes an index listing the topics covered which are an introduction to vaccines, the history of vaccines including the work of Edward Jenner and Louis Pasteur, the production process for vaccines, and applications of specific vaccines for measles, polio, typhoid, hepatitis B, tetanus, and current research on vaccine adherence. The presentation provides overviews of the different vaccines discussed, including dosing schedules, and ends with a list of references.