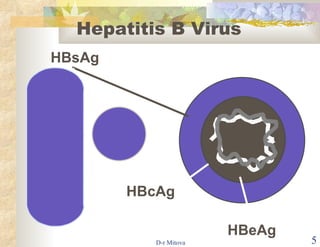
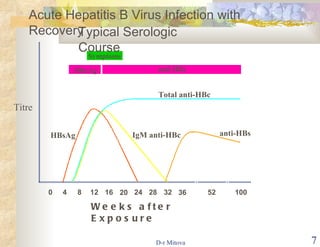
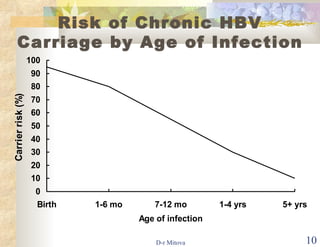
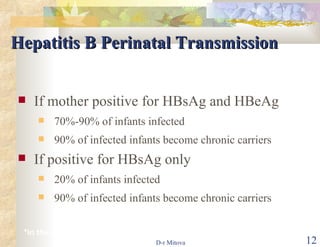
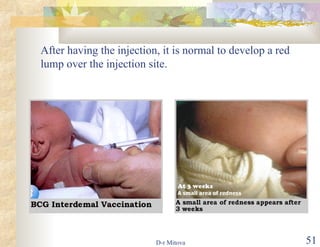
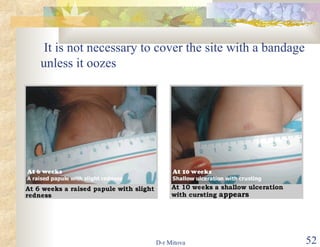
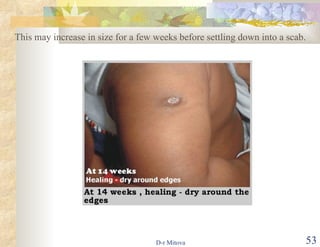
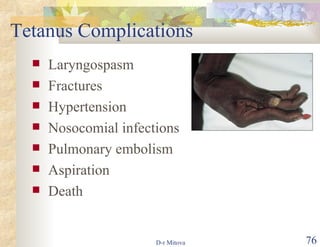
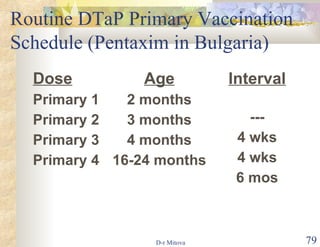
The document discusses hepatitis B virus (HBV) infection and the hepatitis B vaccine. Some key points:
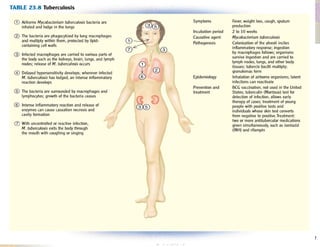
- HBV is a major global health problem that can cause acute and chronic liver disease. It is transmitted through blood and bodily fluids.
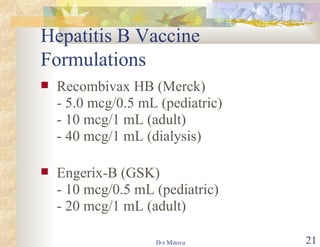
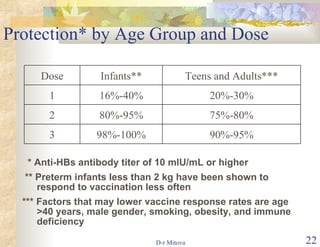
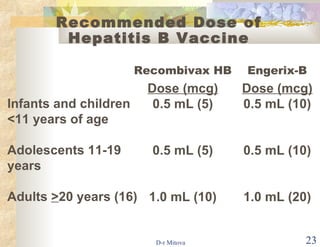
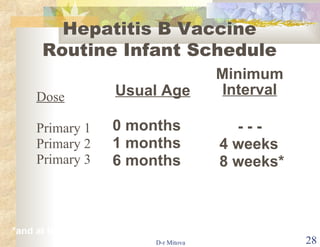
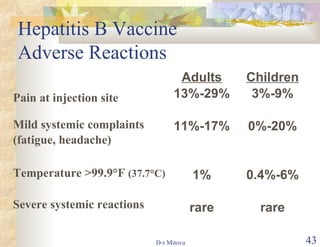
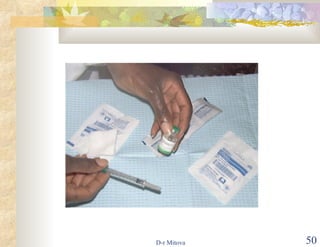
- The hepatitis B vaccine is highly effective and provides long-lasting protection against HBV infection. It has been part of routine infant vaccination programs worldwide since the 1990s.
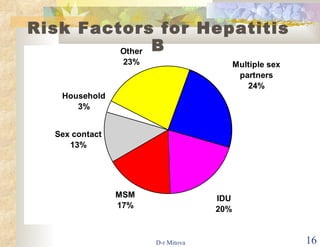
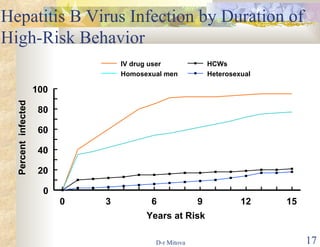
- Vaccination is also recommended for at-risk groups like healthcare workers, injection drug users, and those with multiple sexual partners to prevent HBV transmission.