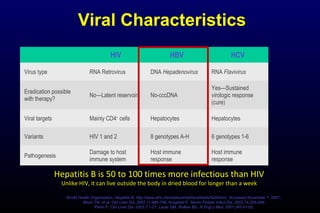
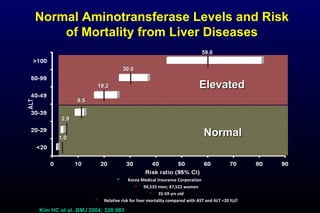
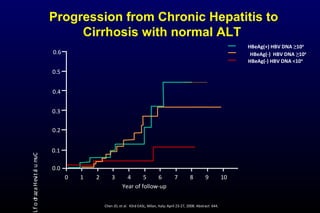
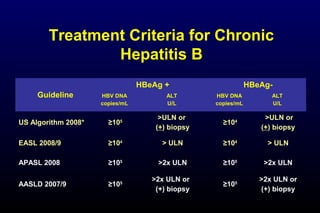
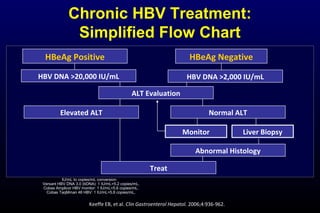
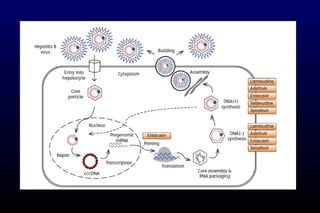
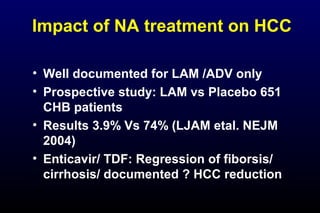
HBV has been associated with humans for over 1,000 years. Recent evidence from a mummified Korean child who tested positive for HBV DNA establishes that HBV has been present in humans for at least 500 years. Treatment guidelines recommend antiviral therapy for patients with chronic HBV based on HBV DNA levels and ALT levels. Tenofovir and entecavir are preferred first-line treatments due to their superior efficacy, tolerability and low resistance profiles. Long-term antiviral therapy can reduce the risk of liver decompensation, hepatocellular carcinoma, and death in patients with chronic HBV.