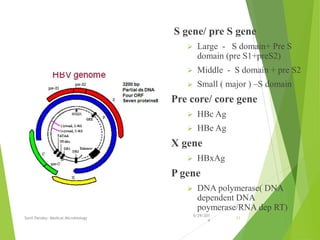
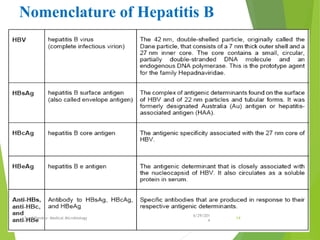
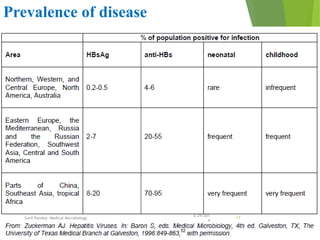
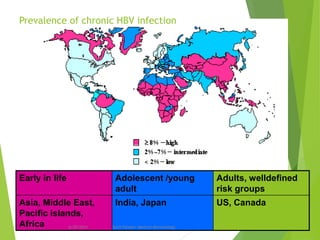
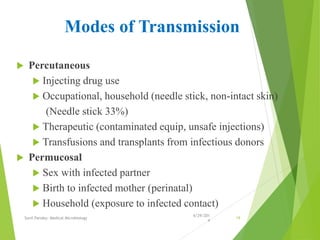
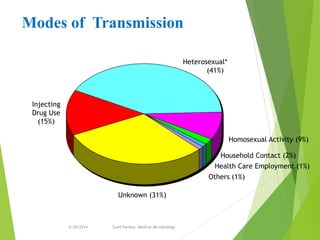
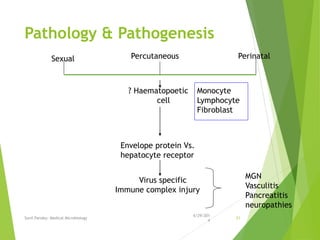
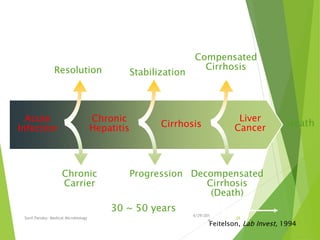
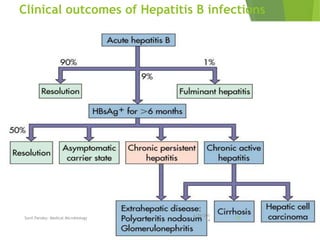
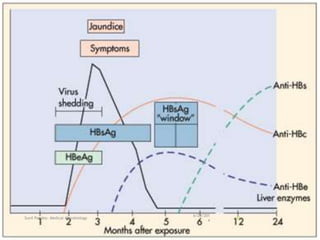
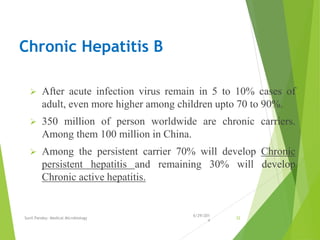
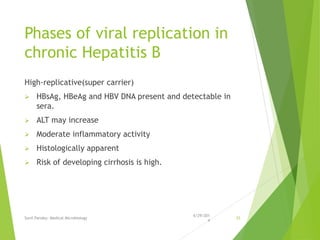
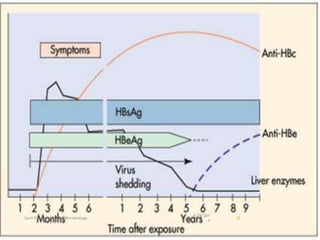
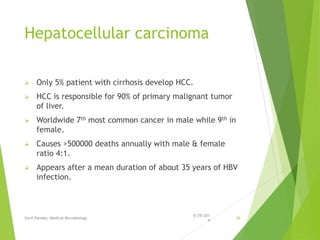
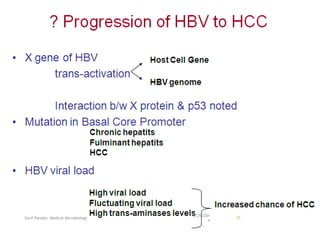
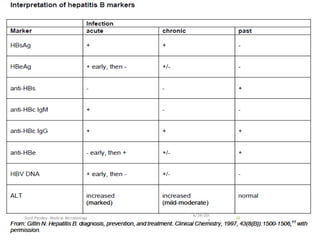
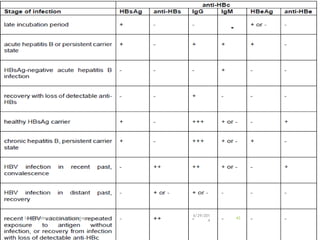
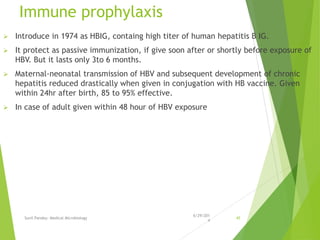
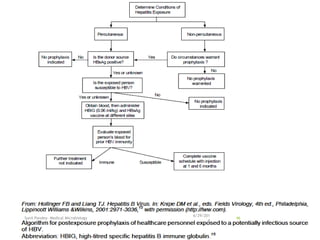
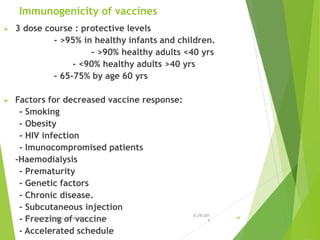
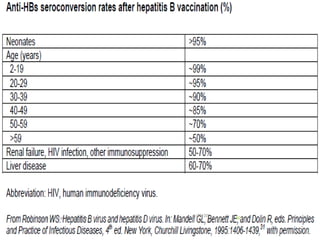
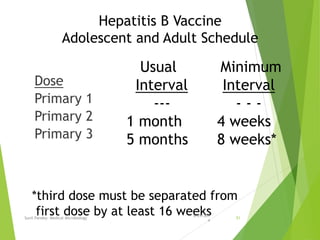
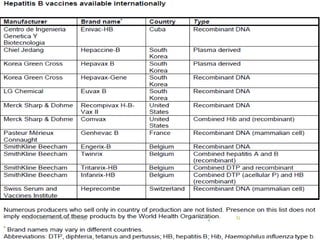
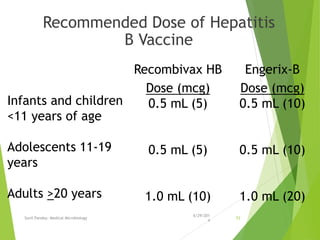
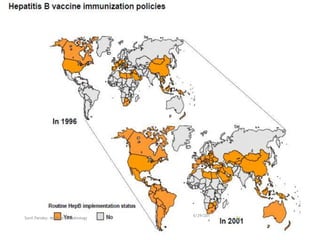
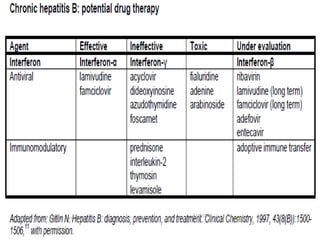
The document provides information about Hepatitis B virus (HBV). It discusses the history of HBV discovery, morphology and structure of the virus, epidemiology, transmission routes, clinical outcomes, and laboratory diagnosis. Key points include that HBV is a partially double-stranded DNA virus that infects hepatocytes and can lead to both acute and chronic liver disease. It remains a major global health problem with 350 million chronic carriers worldwide.