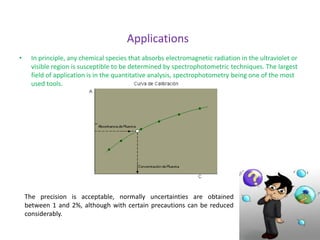
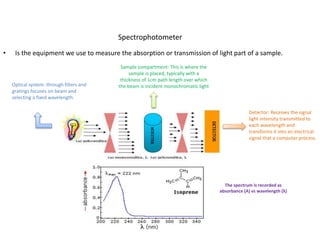
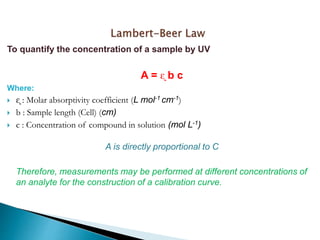
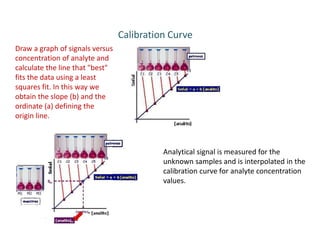
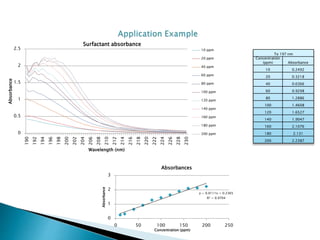
This document describes a study on modifying the properties of wettability and corrosion inhibition for heterogeneous reservoirs using branched zwitterionic gemini liquids. The study involved designing and synthesizing these gemini liquids and evaluating their effects on wettability and corrosion inhibition properties.