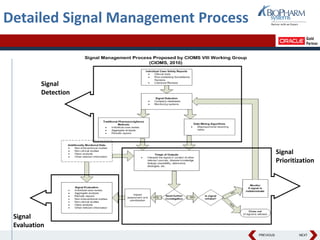
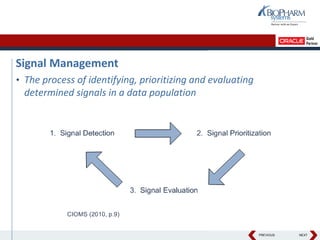
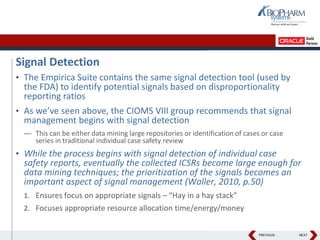
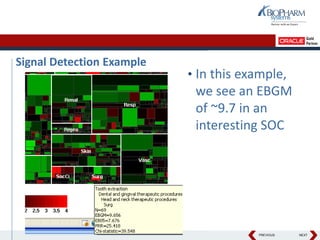
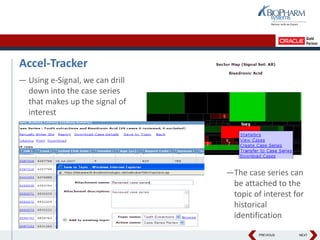
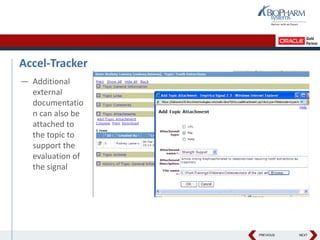
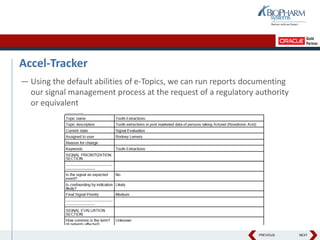
- The document discusses using Oracle's Empirica Topics software to document a company's signal management process.
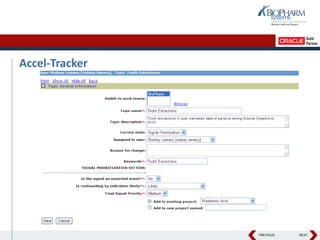
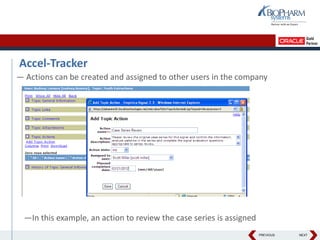
- It describes how the software can be used to detect signals, prioritize signals using standardized questions, and evaluate signals to feed into risk management.
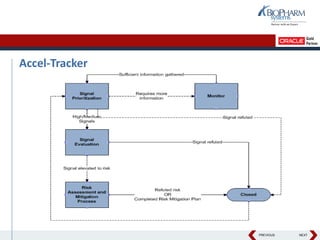
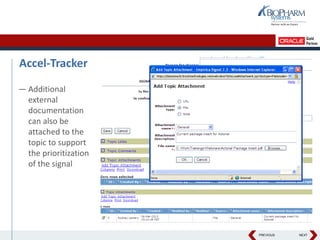
- Specifically, it presents "Accel-Tracker", which is a configuration of Empirica Topics that provides predefined workflows and questions to guide companies through standardized signal detection, prioritization, and evaluation based on industry guidelines.