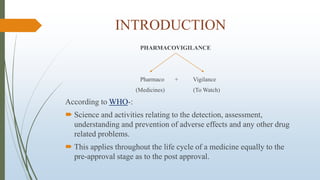
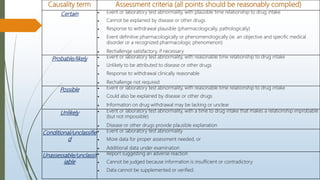
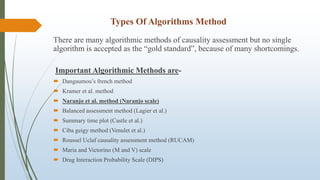
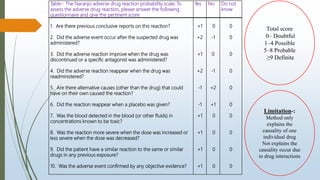
The document provides an overview of causality assessment in pharmacovigilance, which is essential for understanding the relationship between drug treatments and adverse effects. It outlines methods for conducting causality assessments, including expert judgement and various algorithms, along with established criteria for categorizing adverse drug reactions (ADRs). Additionally, it emphasizes the significance of accurately assessing causality to minimize patient suffering and optimize drug safety monitoring.